Prognostic Genetic Markers for Thrombosis in COVID-19 Patients: A Focused Analysis on D-Dimer, Homocysteine and Thromboembolism
- PMID: 33362545
- PMCID: PMC7756688
- DOI: 10.3389/fphar.2020.587451
Prognostic Genetic Markers for Thrombosis in COVID-19 Patients: A Focused Analysis on D-Dimer, Homocysteine and Thromboembolism
Abstract
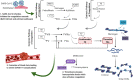
COVID-19 is caused by Severe Acute Respiratory Syndrome Coronavirus-2, which has infected over thirty eight million individuals worldwide. Emerging evidence indicates that COVID-19 patients are at a high risk of developing coagulopathy and thrombosis, conditions that elevate levels of D-dimer. It is believed that homocysteine, an amino acid that plays a crucial role in coagulation, may also contribute to these conditions. At present, multiple genes are implicated in the development of these disorders. For example, single-nucleotide polymorphisms (SNPs) in FGG, FGA, and F5 mediate increases in D-dimer and SNPs in ABO, CBS, CPS1 and MTHFR mediate differences in homocysteine levels, and SNPs in TDAG8 associate with Heparin-induced Thrombocytopenia. In this study, we aimed to uncover the genetic basis of the above conditions by examining genome-wide associations and tissue-specific gene expression to build a molecular network. Based on gene ontology, we annotated various SNPs with five ancestral terms: pulmonary embolism, venous thromboembolism, vascular diseases, cerebrovascular disorders, and stroke. The gene-gene interaction network revealed three clusters that each contained hallmark genes for D-dimer/fibrinogen levels, homocysteine levels, and arterial/venous thromboembolism with F2 and F5 acting as connecting nodes. We propose that genotyping COVID-19 patients for SNPs examined in this study will help identify those at greatest risk of complications linked to thrombosis.
Keywords: COVID-19; coagulopathy; d-dimer; heparin; homocysteine; pulmonary embolism; thrombocytopenia; venous thromboembolism.
Copyright © 2020 Abu-Farha, Al-Sabah, Hammad, Hebbar, Channanath, John, Taher, Almaeen, Ghazy, Mohammad, Abubaker, Arefanian, Al-Mulla and Thanaraj.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

