Mitochondrial Fission and Mitophagy Coordinately Restrict High Glucose Toxicity in Cardiomyocytes
- PMID: 33362579
- PMCID: PMC7758327
- DOI: 10.3389/fphys.2020.604069
Mitochondrial Fission and Mitophagy Coordinately Restrict High Glucose Toxicity in Cardiomyocytes
Abstract
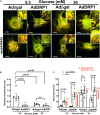
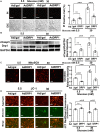
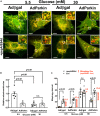
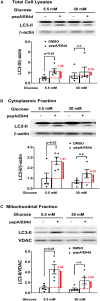
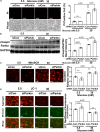
Hyperglycemia-induced mitochondrial dysfunction plays a key role in the pathogenesis of diabetic cardiomyopathy. Injured mitochondrial segments are separated by mitochondrial fission and eliminated by autophagic sequestration and subsequent degradation in the lysosome, a process termed mitophagy. However, it remains poorly understood how high glucose affects the activities of, and the relationship between, mitochondrial fission and mitophagy in cardiomyocytes. In this study, we determined the functional roles of mitochondrial fission and mitophagy in hyperglycemia-induced cardiomyocyte injury. High glucose (30 mM, HG) reduced mitochondrial connectivity and particle size and increased mitochondrial number in neonatal rat ventricular cardiomyocytes, suggesting an enhanced mitochondrial fragmentation. SiRNA knockdown of the pro-fission factor dynamin-related protein 1 (DRP1) restored mitochondrial size but did not affect HG toxicity, and Mdivi-1, a DRP1 inhibitor, even increased HG-induced cardiomyocyte injury, as shown by superoxide production, mitochondrial membrane potential and cell death. However, DRP1 overexpression triggered mitochondrial fragmentation and mitigated HG-induced cardiomyocyte injury, suggesting that the increased mitochondrial fission is beneficial, rather than detrimental, to cardiomyocytes cultured under HG conditions. This is in contrast to the prevailing hypothesis that mitochondrial fragmentation mediates or contributes to HG cardiotoxicity. Meanwhile, HG reduced mitophagy flux as determined by the difference in the levels of mitochondria-associated LC3-II or the numbers of mitophagy foci indicated by the novel dual fluorescent reporter mt-Rosella in the absence and presence of the lysosomal inhibitors. The ability of HG to induce mitochondrial fragmentation and inhibit mitophagy was reproduced in adult mouse cardiomyocytes. Overexpression of Parkin, a positive regulator of mitophagy, or treatment with CCCP, a mitochondrial uncoupler, induced mitophagy and attenuated HG-induced cardiomyocyte death, while Parkin knockdown had opposite effects, suggesting an essential role of mitophagy in cardiomyocyte survival under HG conditions. Strikingly, Parkin overexpression increased mitochondrial fragmentation, while DRP1 overexpression accelerated mitophagy flux, demonstrating a reciprocal activation loop that controls mitochondrial fission and mitophagy. Thus, strategies that promote the mutual positive interaction between mitochondrial fission and mitophagy while simultaneously maintain their levels within the physiological range would be expected to improve mitochondrial health, alleviating hyperglycemic cardiotoxicity.
Keywords: DRP1; Parkin; cardiomyocytes; cell death; diabetes; hyperglycemia; mitochondrial fission; mitophagy.
Copyright © 2020 Kobayashi, Zhao, Zhang, Kobayashi, Huang, Shi, Wu and Liang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

