The Progress of Immunotherapy in Refractory Pituitary Adenomas and Pituitary Carcinomas
- PMID: 33362722
- PMCID: PMC7761748
- DOI: 10.3389/fendo.2020.608422
The Progress of Immunotherapy in Refractory Pituitary Adenomas and Pituitary Carcinomas
Abstract
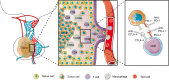
Most pituitary adenomas (PAs) are considered benign tumors, but approximately 0.2% can present metastasis and are classified as pituitary carcinomas (PCs). Refractory PAs lie between benign adenomas and true malignant PC and are defined as aggressive-invasive PAs characterized by a high Ki-67 index, rapid growth, frequent recurrence, and resistance to conventional treatments, including temozolomide. It is notoriously difficult to manage refractory PAs and PC because of the limited therapeutic options. As a promising therapeutic approach, cancer immunotherapy has been experimentally used for the treatment of many tumors, including pituitary tumors. The purpose of this review is to report the progress of immunotherapy in pituitary tumors, including refractory PAs and PCs. The tumor immune microenvironment has been recognized as a key contributor to tumorigenesis, progression, and prognosis. One study indicated that the number of CD68+ macrophages was positively correlated with tumor size and Knosp classification grade for tumor invasiveness. The infiltration of CD4+ and CD8+ T cells was relatively scant in these adenomas, but pituitary growth hormone (GH) adenomas exhibited significantly more CD4+ and CD8+ T cells than non-GH adenomas. These results suggest an association of CD68+ macrophage infiltration with an increase in pituitary tumor size and invasiveness. Another study suggested that a lower number of CD8+ lymphocytes is associated with cavernous sinus invasion and resistance to treatment with first-generation somatostatin analogs in acromegaly patients, highlighting a potential role of the tumor immune microenvironment in determining the prognosis of somatotroph pituitary tumors. Preclinical studies have indicated that widely varying degrees of programmed death-ligand 1 (PD-L1) expression and tumor-infiltrating lymphocytes (TILs) are found among different subtypes. Functional PAs and aggressive PAs express significantly higher levels of PD-L1 and TILs than other subtypes, indicating that PD-1 blockade might be a promising alternative therapy for patients with aggressive PAs. PD-L1 transcript and protein levels were found to be significantly increased in functioning (GH and prolactin-expressing) pituitary tumors compared to nonfunctioning (null cell and silent gonadotroph) adenomas. Moreover, primary pituitary tumors harbored higher levels of PD-L1 mRNA than recurrent tumors. These findings suggest the possibility of considering checkpoint blockade immunotherapy for functioning pituitary tumors refractory to conventional management. Animal models of Cushing's disease also demonstrated PD-L1 and TIL expression in cultured tumors and murine models, as well as the effectiveness of checkpoint blockade therapy in reducing the tumor mass, decreasing hormone secretion, and increasing the survival rate. Clinical studies show that immunotherapy may be an effective treatment in patients with pituitary tumors. One corticotroph carcinoma patient showed a significant reduction in hormone levels and shrinkage of the tumor size of primary and metastatic lesions immediately after investigational treatment with ipilimumab and nivolumab. However, another patient with corticotroph adenoma progressed rapidly after four cycles of anti-PD-1 (pembrolizumab) treatment. To date, there are two registered clinical trials of immunotherapy for pituitary tumors. One of them is the phase II clinical trial of nivolumab combined with ipilimumab for patients with aggressive pituitary tumors (NCT04042753). The other one is also a phase II clinical trial of the combination of nivolumab and ipilimumab for rare tumors, including pituitary tumors (NCT02834013). Both clinical trials are in the stage of recruiting patients and have not been completed. In summary, the results from preclinical research and clinical studies indicated that immunotherapy might be a promising alternative therapy for PCs and refractory PAs resistant to conventional treatments. The combination of immunotherapy and radiotherapy or temozolomide may have synergistic effects compared to a single treatment. More preclinical and clinical studies are needed to further indicate the exact efficacy of immunotherapy in pituitary tumors.
Keywords: immunotherapy; pituitary carcinomas; programmed death-ligand 1; refractory pituitary adenomas; tumor-infiltrating lymphocytes.
Copyright © 2020 Dai, Liang, Sun and Kang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The expression profile of PD-L1 and CD8+ lymphocyte in pituitary adenomas indicating for immunotherapy.J Neurooncol. 2018 Aug;139(1):89-95. doi: 10.1007/s11060-018-2844-2. Epub 2018 Apr 21. J Neurooncol. 2018. PMID: 29680903
-
Increased expression of programmed death ligand 1 (PD-L1) in human pituitary tumors.Oncotarget. 2016 Nov 22;7(47):76565-76576. doi: 10.18632/oncotarget.12088. Oncotarget. 2016. PMID: 27655724 Free PMC article.
-
Anti-VEGF Therapy in Refractory Pituitary Adenomas and Pituitary Carcinomas: A Review.Front Oncol. 2021 Nov 17;11:773905. doi: 10.3389/fonc.2021.773905. eCollection 2021. Front Oncol. 2021. PMID: 34869016 Free PMC article. Review.
-
Immune Cell Infiltrates in Pituitary Adenomas: More Macrophages in Larger Adenomas and More T Cells in Growth Hormone Adenomas.Endocr Pathol. 2015 Sep;26(3):263-72. doi: 10.1007/s12022-015-9383-6. Endocr Pathol. 2015. PMID: 26187094
-
Evolution of a refractory prolactin-secreting pituitary adenoma into a pituitary carcinoma: report of a challenging case and literature review.BMC Endocr Disord. 2021 Oct 29;21(1):217. doi: 10.1186/s12902-021-00874-8. BMC Endocr Disord. 2021. PMID: 34715828 Free PMC article. Review.
Cited by
-
Coexistence of Pituitary Adenoma and Primary Pituitary Lymphoma: A Case Report and Review of the Literature.Front Surg. 2022 Mar 16;9:842830. doi: 10.3389/fsurg.2022.842830. eCollection 2022. Front Surg. 2022. PMID: 35372490 Free PMC article.
-
PD-L1 expression in PitNETs: Correlations with the 2022 WHO classification.Brain Spine. 2024 Dec 24;5:104171. doi: 10.1016/j.bas.2024.104171. eCollection 2025. Brain Spine. 2024. PMID: 39845357 Free PMC article.
-
The immune microenviroment in somatotropinomas: from biology to personalized and target therapy.Rev Endocr Metab Disord. 2023 Apr;24(2):283-295. doi: 10.1007/s11154-022-09782-1. Epub 2023 Jan 20. Rev Endocr Metab Disord. 2023. PMID: 36658300 Free PMC article. Review.
-
Exploring the role of the tumor microenvironment in refractory pituitary tumor pathogenesis.Pituitary. 2023 Jun;26(3):263-265. doi: 10.1007/s11102-023-01301-y. Epub 2023 Mar 4. Pituitary. 2023. PMID: 36870010 Review.
-
Immune Checkpoints: Therapeutic Targets for Pituitary Tumors.Dis Markers. 2021 Aug 16;2021:5300381. doi: 10.1155/2021/5300381. eCollection 2021. Dis Markers. 2021. PMID: 34447484 Free PMC article. Review.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials