Early Phases of COVID-19 Are Characterized by a Reduction in Lymphocyte Populations and the Presence of Atypical Monocytes
- PMID: 33362757
- PMCID: PMC7756112
- DOI: 10.3389/fimmu.2020.560330
Early Phases of COVID-19 Are Characterized by a Reduction in Lymphocyte Populations and the Presence of Atypical Monocytes
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 is a recently discovered pathogen responsible of coronavirus disease 2019 (COVID-19). The immunological changes associated with this infection are largely unknown.
Methods: We evaluated the peripheral blood mononuclear cells profile of 63 patients with COVID-19 at diagnosis. We also assessed the presence of association with inflammatory biomarkers and the 28-day mortality.
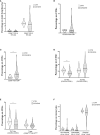
Results: Lymphocytopenia was present in 51 of 63 (80.9%) patients, with a median value of 720 lymphocytes/µl (IQR 520-1,135). This reduction was mirrored also on CD8+ (128 cells/µl, IQR 55-215), natural killer (67 cells/µl, IQR 35-158) and natural killer T (31 cells/µl, IQR 11-78) cells. Monocytes were preserved in total number but displayed among them a subpopulation with a higher forward and side scatter properties, composed mainly of cells with a reduced expression of both CD14 and HLA-DR. Patients who died in the 28 days from admission (N=10, 15.9%), when compared to those who did not, displayed lower mean values of CD3+ (337.4 cells/µl vs 585.9 cells/µl; p=0.028) and CD4+ cells (232.2 cells/µl vs 381.1 cells/µl; p=0.042) and an higher percentage of CD8+/CD38+/HLA-DR+ lymphocytes (13.5% vs 7.6%; p=0.026).
Discussion: The early phases of COVID-19 are characterized by lymphocytopenia, predominance of Th2-like lymphocytes and monocytes with altered immune profile, which include atypical mononuclear cells.
Keywords: COVID-19; SARS-CoV-2; immune profiling; inflammation; monocytes; peripheral blood mononuclear cells.
Copyright © 2020 Lombardi, Trombetta, Cattaneo, Castelli, Palomba, Tirone, Mangioni, Lamorte, Manunta, Prati, Ceriotti, Gualtierotti, Costantino, Aliberti, Scaravilli, Grasselli, Gori, Porretti and Bandera.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- World Health Organization (WHO) Coronavirus disease 2019 (COVID-19) Situation Report - 72. (2020). https://pers.droneemprit.id/covid19/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

