Neutrophil Inflammatory Response Is Downregulated by Uptake of Superparamagnetic Iron Oxide Nanoparticle Therapeutics
- PMID: 33362760
- PMCID: PMC7757401
- DOI: 10.3389/fimmu.2020.571489
Neutrophil Inflammatory Response Is Downregulated by Uptake of Superparamagnetic Iron Oxide Nanoparticle Therapeutics
Abstract
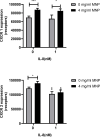
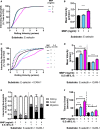
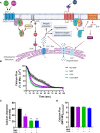
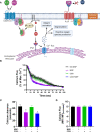
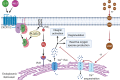
Superparamagnetic iron oxide nanoparticles (SPION) are employed as diagnostics and therapeutics following intravenous delivery for the treatment of iron deficiency anemia (IDA) in adult patients with chronic kidney failure. Neutrophils are the first defense against blood borne foreign insult and recruit to vascular sites of inflammation via a sequential process that is characterized by adhesive capture, rolling, and shear resistant arrest. A primary chemotactic agonist presented on the glycocalyx of inflamed endothelium is IL-8, which upon binding to its cognate membrane receptor (CXCR1/2) activates a suite of responses in neutrophils. An early response is degranulation with accompanying upregulation of β2-integrin (CD11/CD18) and shedding of L-selectin (CD62L) receptors, which exert differential effects on the efficiency of endothelial recruitment. Feraheme is an FDA approved SPION treatment for IDA, but its effect on the innate immune response of neutrophils during inflammation has not been reported. Here, we studied the immunomodulatory effects of Feraheme on neutrophils freshly isolated from healthy human subjects and stimulated in suspension or on inflammatory mimetic substrates with IL-8. Cells treated with Feraheme exhibited reduced sensitivity to stimulation with IL-8, indicated by reduced upregulation of membrane CD11b/CD18 receptors, high affinity (HA) CD18, and shedding of CD62L. Feraheme also inhibited N-formyl-Met-Leu-Phe (fMLP) induced reactive oxygen species production. Neutrophil rolling, arrest, and migration was assessed in vascular mimetic microfluidic channels coated with E-selectin and ICAM-1 to simulate inflamed endothelium. Neutrophils exposed to Feraheme rolled faster on E-selectin and arrested less frequently on ICAM-1, in a manner dependent upon SPION concentration. Subsequent neutrophil shape change, and migration were also significantly inhibited in the presence of Feraheme. Lastly, Feraheme accelerated clearance of cytosolic calcium flux following IL-8 stimulation. We conclude that uptake of Feraheme by neutrophils inhibits chemotactic activation and downregulates normal rolling to arrest under shear flow. The mechanism involves increased calcium clearance following chemotactic activation, which may diminish the efficiency of recruitment from the circulation at vascular sites of inflammation.
Keywords: immunosupression; inflammation; innate immunity; iron oxide; mechanosignaling; nanoparticle; neutrophil degranulation; neutrophil recruitment.
Copyright © 2020 Garcia, Kim, Morikis and Simon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Neutrophil CD18-dependent arrest on intercellular adhesion molecule 1 (ICAM-1) in shear flow can be activated through L-selectin.J Immunol. 1997 Jan 1;158(1):367-75. J Immunol. 1997. PMID: 8977212
-
Calcium flux in neutrophils synchronizes beta2 integrin adhesive and signaling events that guide inflammatory recruitment.Ann Biomed Eng. 2008 Apr;36(4):632-46. doi: 10.1007/s10439-008-9453-8. Epub 2008 Feb 16. Ann Biomed Eng. 2008. PMID: 18278555 Free PMC article.
-
Shear-dependent capping of L-selectin and P-selectin glycoprotein ligand 1 by E-selectin signals activation of high-avidity beta2-integrin on neutrophils.J Immunol. 2004 Jun 15;172(12):7780-90. doi: 10.4049/jimmunol.172.12.7780. J Immunol. 2004. PMID: 15187162
-
Leukocyte-endothelial cell interactions.Semin Hematol. 1993 Oct;30(4 Suppl 4):45-53; discussion 54-5. Semin Hematol. 1993. PMID: 8303310 Review.
-
E-selectin ligands as mechanosensitive receptors on neutrophils in health and disease.Ann Biomed Eng. 2012 Apr;40(4):849-59. doi: 10.1007/s10439-011-0507-y. Epub 2012 Jan 24. Ann Biomed Eng. 2012. PMID: 22271244 Free PMC article. Review.
Cited by
-
A Novel Experimental Approach to Understand the Transport of Nanodrugs.Materials (Basel). 2023 Aug 5;16(15):5485. doi: 10.3390/ma16155485. Materials (Basel). 2023. PMID: 37570188 Free PMC article.
-
Polypropylene nanoplastic exposure leads to lung inflammation through p38-mediated NF-κB pathway due to mitochondrial damage.Part Fibre Toxicol. 2023 Jan 10;20(1):2. doi: 10.1186/s12989-022-00512-8. Part Fibre Toxicol. 2023. PMID: 36624477 Free PMC article.
-
New Concepts on the Pathophysiology of Acute Coronary Syndrome.Rev Cardiovasc Med. 2023 Apr 17;24(4):112. doi: 10.31083/j.rcm2404112. eCollection 2023 Apr. Rev Cardiovasc Med. 2023. PMID: 39076267 Free PMC article. Review.
-
Neutrophil Extracellular Trap-Driven Occlusive Diseases.Cells. 2021 Aug 26;10(9):2208. doi: 10.3390/cells10092208. Cells. 2021. PMID: 34571857 Free PMC article. Review.
-
Nanoparticles in the diagnosis and treatment of vascular aging and related diseases.Signal Transduct Target Ther. 2022 Jul 11;7(1):231. doi: 10.1038/s41392-022-01082-z. Signal Transduct Target Ther. 2022. PMID: 35817770 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

