IgE and IgG Antibodies as Regulators of Mast Cell and Basophil Functions in Food Allergy
- PMID: 33362785
- PMCID: PMC7759531
- DOI: 10.3389/fimmu.2020.603050
IgE and IgG Antibodies as Regulators of Mast Cell and Basophil Functions in Food Allergy
Abstract
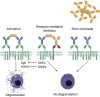
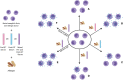
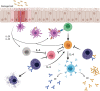
Food allergy is a major health issue, affecting the lives of 8% of U.S. children and their families. There is an urgent need to identify the environmental and endogenous signals that induce and sustain allergic responses to ingested allergens. Acute reactions to foods are triggered by the activation of mast cells and basophils, both of which release inflammatory mediators that lead to a range of clinical manifestations, including gastrointestinal, cutaneous, and respiratory reactions as well as systemic anaphylaxis. Both of these innate effector cell types express the high affinity IgE receptor, FcϵRI, on their surface and are armed for adaptive antigen recognition by very-tightly bound IgE antibodies which, when cross-linked by polyvalent allergen, trigger degranulation. These cells also express inhibitory receptors, including the IgG Fc receptor, FcγRIIb, that suppress their IgE-mediated activation. Recent studies have shown that natural resolution of food allergies is associated with increasing food-specific IgG levels. Furthermore, oral immunotherapy, the sequential administration of incrementally increasing doses of food allergen, is accompanied by the strong induction of allergen-specific IgG antibodies in both human subjects and murine models. These can deliver inhibitory signals via FcγRIIb that block IgE-induced immediate food reactions. In addition to their role in mediating immediate hypersensitivity reactions, mast cells and basophils serve separate but critical functions as adjuvants for type 2 immunity in food allergy. Mast cells and basophils, activated by IgE, are key sources of IL-4 that tilts the immune balance away from tolerance and towards type 2 immunity by promoting the induction of Th2 cells along with the innate effectors of type 2 immunity, ILC2s, while suppressing the development of regulatory T cells and driving their subversion to a pathogenic pro-Th2 phenotype. This adjuvant effect of mast cells and basophils is suppressed when inhibitory signals are delivered by IgG antibodies signaling via FcγRIIb. This review summarizes current understanding of the immunoregulatory effects of mast cells and basophils and how these functions are modulated by IgE and IgG antibodies. Understanding these pathways could provide important insights into innovative strategies for preventing and/or reversing food allergy in patients.
Keywords: Fc receptor; IgE; IgG; basophil activation test; food allergy; mast cells; oral immunotherapy.
Copyright © 2020 Kanagaratham, El Ansari, Lewis and Oettgen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

