All About the RNA: Interferon-Stimulated Genes That Interfere With Viral RNA Processes
- PMID: 33362792
- PMCID: PMC7756014
- DOI: 10.3389/fimmu.2020.605024
All About the RNA: Interferon-Stimulated Genes That Interfere With Viral RNA Processes
Abstract
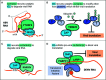
Interferon (IFN) signaling induces the expression of a wide array of genes, collectively referred to as IFN-stimulated genes (ISGs) that generally function to inhibit viral replication. RNA viruses are frequently targeted by ISGs through recognition of viral replicative intermediates and molecular features associated with viral genomes, or the lack of molecular features associated with host mRNAs. The ISGs reviewed here primarily inhibit viral replication in an RNA-centric manner, working to sense, degrade, or repress expression of viral RNA. This review focuses on dissecting how these ISGs exhibit multiple antiviral mechanisms, often through use of varied co-factors, highlighting the complexity of the type I IFN response. Specifically, these ISGs can mediate antiviral effects through viral RNA degradation, viral translation inhibition, or both. While the OAS/RNase L pathway globally degrades RNA and arrests translation, ISG20 and ZAP employ targeted RNA degradation and translation inhibition to block viral replication. Meanwhile, SHFL targets translation by inhibiting -1 ribosomal frameshifting, which is required by many RNA viruses. Finally, a number of E3 ligases inhibit viral transcription, an attractive antiviral target during the lifecycle of negative-sense RNA viruses which must transcribe their genome prior to translation. Through this review, we aim to provide an updated perspective on how these ISGs work together to form a complex network of antiviral arsenals targeting viral RNA processes.
Keywords: RNA sensing; co-factors; interferon-stimulated genes; translation inhibition; viral RNA degradation.
Copyright © 2020 Yang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

