Importance of Type I and III Interferons at Respiratory and Intestinal Barrier Surfaces
- PMID: 33362795
- PMCID: PMC7759678
- DOI: 10.3389/fimmu.2020.608645
Importance of Type I and III Interferons at Respiratory and Intestinal Barrier Surfaces
Abstract
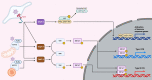
Interferons (IFNs) constitute the first line of defense against microbial infections particularly against viruses. They provide antiviral properties to cells by inducing the expression of hundreds of genes known as interferon-stimulated genes (ISGs). The two most important IFNs that can be produced by virtually all cells in the body during intrinsic innate immune response belong to two distinct families: the type I and type III IFNs. The type I IFN receptor is ubiquitously expressed whereas the type III IFN receptor's expression is limited to epithelial cells and a subset of immune cells. While originally considered to be redundant, type III IFNs have now been shown to play a unique role in protecting mucosal surfaces against pathogen challenges. The mucosal specific functions of type III IFN do not solely rely on the restricted epithelial expression of its receptor but also on the distinct means by which type III IFN mediates its anti-pathogen functions compared to the type I IFN. In this review we first provide a general overview on IFNs and present the similarities and differences in the signal transduction pathways leading to the expression of either type I or type III IFNs. By highlighting the current state-of-knowledge of the two archetypical mucosal surfaces (e.g. the respiratory and intestinal epitheliums), we present the differences in the signaling cascades used by type I and type III IFNs to uniquely induce the expression of ISGs. We then discuss in detail the role of each IFN in controlling pathogen infections in intestinal and respiratory epithelial cells. Finally, we provide our perspective on novel concepts in the field of IFN (stochasticity, response heterogeneity, cellular polarization/differentiation and tissue microenvironment) that we believe have implications in driving the differences between type I and III IFNs and could explain the preferences for type III IFNs at mucosal surfaces.
Keywords: epithelial cells; interferon lambda; interferons; intestinal epithelium; mucosal immunity; respiratory epithelia; type I interferon; type III interferon.
Copyright © 2020 Stanifer, Guo, Doldan and Boulant.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

