Tolerance to Bone Marrow Transplantation: Do Mesenchymal Stromal Cells Still Have a Future for Acute or Chronic GvHD?
- PMID: 33362797
- PMCID: PMC7759493
- DOI: 10.3389/fimmu.2020.609063
Tolerance to Bone Marrow Transplantation: Do Mesenchymal Stromal Cells Still Have a Future for Acute or Chronic GvHD?
Abstract
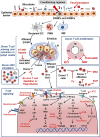
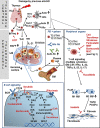
Mesenchymal Stromal Cells (MSCs) are fibroblast-like cells of mesodermal origin present in many tissues and which have the potential to differentiate to osteoblasts, adipocytes and chondroblasts. They also have a clear immunosuppressive and tissue regeneration potential. Indeed, the initial classification of MSCs as pluripotent stem cells, has turned into their identification as stromal progenitors. Due to the relatively simple procedures available to expand in vitro large numbers of GMP grade MSCs from a variety of different tissues, many clinical trials have tested their therapeutic potential in vivo. One pathological condition where MSCs have been quite extensively tested is steroid resistant (SR) graft versus host disease (GvHD), a devastating condition that may occur in acute or chronic form following allogeneic hematopoietic stem cell transplantation. The clinical and experimental results obtained have outlined a possible efficacy of MSCs, but unfortunately statistical significance in clinical studies has only rarely been reached and effects have been relatively limited in most cases. Nonetheless, the extremely complex pathogenetic mechanisms at the basis of GvHD, the fact that studies have been conducted often in patients who had been previously treated with multiple lines of therapy, the variable MSC doses and schedules administered in different trials, the lack of validated potency assays and clear biomarkers, the difference in MSC sources and production methods may have been major factors for this lack of clear efficacy in vivo. The heterogeneity of MSCs and their different stromal differentiation potential and biological activity may be better understood through more refined single cell sequencing and proteomic studies, where either an "anti-inflammatory" or a more "immunosuppressive" profile can be identified. We summarize the pathogenic mechanisms of acute and chronic GvHD and the role for MSCs. We suggest that systematic controlled clinical trials still need to be conducted in the most promising clinical settings, using better characterized cells and measuring efficacy with specific biomarkers, before strong conclusions can be drawn about the therapeutic potential of these cells in this context. The same analysis should be applied to other inflammatory, immune or degenerative diseases where MSCs may have a therapeutic potential.
Keywords: graft versus host disease; hematopoietic stem cell transplantation; immunosuppressive drugs; inflammation; mesenchymal stromal cell.
Copyright © 2020 Introna and Golay.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Mesenchymal Stromal Cells for Enhancing Hematopoietic Engraftment and Treatment of Graft-Versus-Host Disease, Hemorrhages and Acute Respiratory Distress Syndrome.Front Immunol. 2022 Mar 18;13:839844. doi: 10.3389/fimmu.2022.839844. eCollection 2022. Front Immunol. 2022. PMID: 35371003 Free PMC article.
-
Mesenchymal Stromal Cells in Pediatric Hematopoietic Cell Transplantation a Review and a Pilot Study in Children Treated With Decidua Stromal Cells for Acute Graft-versus-Host Disease.Front Immunol. 2020 Oct 19;11:567210. doi: 10.3389/fimmu.2020.567210. eCollection 2020. Front Immunol. 2020. PMID: 33193339 Free PMC article.
-
Chimerism of bone marrow mesenchymal stem/stromal cells in allogeneic hematopoietic cell transplantation: is it clinically relevant?Chimerism. 2013 Jul-Sep;4(3):78-83. doi: 10.4161/chim.25609. Epub 2013 Jul 11. Chimerism. 2013. PMID: 23880502 Free PMC article. Review.
-
Effects of bone marrow mesenchymal stem cells on hematopoietic recovery and acute graft-versus-host disease in murine allogeneic umbilical cord blood transplantation model.Cell Biochem Biophys. 2014 Sep;70(1):115-22. doi: 10.1007/s12013-014-9866-y. Cell Biochem Biophys. 2014. PMID: 24696072
-
Mesenchymal stromal cells fail to prevent acute graft-versus-host disease and graft rejection after dog leukocyte antigen-haploidentical bone marrow transplantation.Biol Blood Marrow Transplant. 2011 Feb;17(2):214-25. doi: 10.1016/j.bbmt.2010.08.015. Epub 2010 Oct 30. Biol Blood Marrow Transplant. 2011. PMID: 20816818 Free PMC article.
Cited by
-
Novel Treatment for Graft-versus-Host Disease.Blood Cell Ther. 2021 Nov 25;4(4):101-109. doi: 10.31547/bct-2021-022. eCollection 2021 Nov 25. Blood Cell Ther. 2021. PMID: 36714067 Free PMC article.
-
Novel therapies for graft versus host disease with a focus on cell therapies.Front Immunol. 2023 Oct 5;14:1241068. doi: 10.3389/fimmu.2023.1241068. eCollection 2023. Front Immunol. 2023. PMID: 37868964 Free PMC article. Review.
-
Potency assays and biomarkers for cell-based advanced therapy medicinal products.Front Immunol. 2023 Jun 9;14:1186224. doi: 10.3389/fimmu.2023.1186224. eCollection 2023. Front Immunol. 2023. PMID: 37359560 Free PMC article. Review.
-
Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications.J Biomed Sci. 2021 Apr 14;28(1):28. doi: 10.1186/s12929-021-00725-7. J Biomed Sci. 2021. PMID: 33849537 Free PMC article. Review.
-
Revisiting the Mesenchymal "Stem vs. Stromal" Cell Dichotomy and Its Implications for Development of Improved Potency Metrics.Stem Cells. 2023 May 15;41(5):444-452. doi: 10.1093/stmcls/sxad019. Stem Cells. 2023. PMID: 36891977 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

