Abscisic Acid Deficiency Alters Epicuticular Wax Metabolism and Morphology That Leads to Increased Cuticle Permeability During Sweet Orange (Citrus sinensis) Fruit Ripening
- PMID: 33362823
- PMCID: PMC7755607
- DOI: 10.3389/fpls.2020.594184
Abscisic Acid Deficiency Alters Epicuticular Wax Metabolism and Morphology That Leads to Increased Cuticle Permeability During Sweet Orange (Citrus sinensis) Fruit Ripening
Abstract
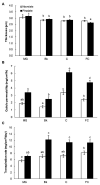
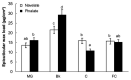
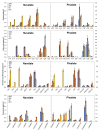
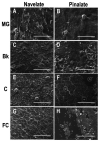
Citrus fruit ripening is coupled with the synthesis and deposition of epicuticular waxes, which reduces water loss during fruit postharvest storage. Although abscisic acid (ABA) is a major regulator of citrus fruit ripening, whether ABA mediates epicuticular wax formation during this process remains poorly understood. We investigated the implication of ABA in cuticle properties and epicuticular wax metabolism, composition, and morphology by comparing the Navelate orange [Citrus sinensis (L.) Osbeck] and its ABA biosynthesis-impaired mutant Pinalate in four ripening stages. ABA deficiency had minor effects on cuticle thickness and epicuticular wax load, but correlated with cuticle permeability. ABA content aligned with mostly fatty acids accumulation in both cultivars, and also with specific alkane, terpenoid, and aldehyde constituents in the parental fruit. In turn, cuticle permeability correlated with the fatty acid profile during fruit ripening in the Navelate and Pinalate, and with primary alcohols, terpenoids, and aldehydes, but only in the mutant fruit. Low ABA levels increased the susceptibility of waxes to crack and were lost from the epicuticular layer. The RNA-seq analysis highlighted the differential regulation of a list of 87 cuticle-related genes between genotypes and ripening stages. Changes in the gene expression of the selected genes in both cultivars were consistent with the content of the aliphatics and terpenoid fractions during ripening. The results suggest a role for ABA in the regulation of fatty acid content and primary alcohol composition, and point out the importance of alkane and triterpenoid for controlling water permeance through fruit cuticles.
Keywords: abscisic acid; cuticle; fruit quality; permeability; ripening; transcriptome; transpiration rate; wax morphology.
Copyright © 2020 Romero and Lafuente.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Albrigo L. G. (1972). Distribution of stomata and epicuticular wax oranges as related to stem end rind breakdown and water loss. J. Am. Soc. Hortic. Sci. 97, 220–223.
-
- Alférez F., Sala J. M., Sanchez-Ballesta M. T., Mulas M., Lafuente M. T., Zacarias L. (2005). A comparative study of the postharvest performance of an ABA-deficient mutant of oranges: I. physiological and quality aspects. Postharvest Biol. Technol. 37, 222–231. 10.1016/j.postharvbio.2005.05.010 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

