Role of Autophagy in the Microenvironment of Oral Squamous Cell Carcinoma
- PMID: 33363032
- PMCID: PMC7756113
- DOI: 10.3389/fonc.2020.602661
Role of Autophagy in the Microenvironment of Oral Squamous Cell Carcinoma
Abstract
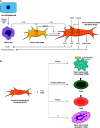
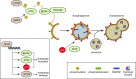
Oral squamous cell carcinoma, the most common type of oral cancer, affects more than 275,000 people per year worldwide. Oral squamous cell carcinoma is very aggressive, as most patients die after 3 to 5 years post-diagnosis. The initiation and progression of oral squamous cell carcinoma are multifactorial: smoking, alcohol consumption, and human papilloma virus infection are among the causes that promote its development. Although oral squamous cell carcinoma involves abnormal growth and migration of oral epithelial cells, other cell types such as fibroblasts and immune cells form the carcinoma niche. An underlying inflammatory state within the oral tissue promotes differential stress-related responses that favor oral squamous cell carcinoma. Autophagy is an intracellular degradation process that allows cancer cells to survive under stress conditions. Autophagy degrades cellular components by sequestering them in vesicles called autophagosomes, which ultimately fuse with lysosomes. Although several autophagy markers have been associated with oral squamous cell carcinoma, it remains unclear whether up- or down-regulation of autophagy favors its progression. Autophagy levels during oral squamous cell carcinoma are both timing- and cell-specific. Here we discuss how autophagy is required to establish a new cellular microenvironment in oral squamous cell carcinoma and how autophagy drives the phenotypic change of oral squamous cell carcinoma cells by promoting crosstalk between carcinoma cells, fibroblasts, and immune cells.
Keywords: autophagy; cancer; carcinoma-associated fibroblast; oral squamous cell carcinoma; tumor microenvironment.
Copyright © 2020 Peña-Oyarzún, Reyes, Hernández-Cáceres, Kretschmar, Morselli, Ramirez-Sarmiento, Lavandero, Torres and Criollo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

