Role of Specific B-Cell Receptor Antigens in Lymphomagenesis
- PMID: 33363034
- PMCID: PMC7756126
- DOI: 10.3389/fonc.2020.604685
Role of Specific B-Cell Receptor Antigens in Lymphomagenesis
Abstract
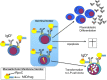
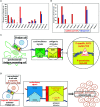
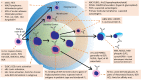
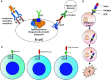
The B-cell receptor (BCR) signaling pathway is a crucial pathway of B cells, both for their survival and for antigen-mediated activation, proliferation and differentiation. Its activation is also critical for the genesis of many lymphoma types. BCR-mediated lymphoma proliferation may be caused by activating BCR-pathway mutations and/or by active or tonic stimulation of the BCR. BCRs of lymphomas have frequently been described as polyreactive. In this review, the role of specific target antigens of the BCRs of lymphomas is highlighted. These antigens have been found to be restricted to specific lymphoma entities. The antigens can be of infectious origin, such as H. pylori in gastric MALT lymphoma or RpoC of M. catarrhalis in nodular lymphocyte predominant Hodgkin lymphoma, or they are autoantigens. Examples of such autoantigens are the BCR itself in chronic lymphocytic leukemia, LRPAP1 in mantle cell lymphoma, hyper-N-glycosylated SAMD14/neurabin-I in primary central nervous system lymphoma, hypo-phosphorylated ARS2 in diffuse large B-cell lymphoma, and hyper-phosphorylated SLP2, sumoylated HSP90 or saposin C in plasma cell dyscrasia. Notably, atypical posttranslational modifications are often responsible for the immunogenicity of many autoantigens. Possible therapeutic approaches evolving from these specific antigens are discussed.
Keywords: B-cell receptor; antigen; antigens of infectious origin; autoreactivity; lymphoma; posttransnational modification.
Copyright © 2020 Thurner, Hartmann, Neumann, Hoth, Stilgenbauer, Küppers, Preuss and Bewarder.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

