Accelerator or Brake: Immune Regulators in Malaria
- PMID: 33363057
- PMCID: PMC7758250
- DOI: 10.3389/fcimb.2020.610121
Accelerator or Brake: Immune Regulators in Malaria
Abstract
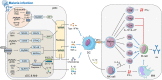
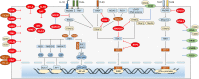
Malaria is a life-threatening infectious disease, affecting over 250 million individuals worldwide each year, eradicating malaria has been one of the greatest challenges to public health for a century. Growing resistance to anti-parasitic therapies and lack of effective vaccines are major contributing factors in controlling this disease. However, the incomplete understanding of parasite interactions with host anti-malaria immunity hinders vaccine development efforts to date. Recent studies have been unveiling the complexity of immune responses and regulators against Plasmodium infection. Here, we summarize our current understanding of host immune responses against Plasmodium-derived components infection and mainly focus on the various regulatory mechanisms mediated by recent identified immune regulators orchestrating anti-malaria immunity.
Keywords: immune regulators; immune responses; malaria; protective immunity; signaling mechanisms; type I interferon.
Copyright © 2020 Cai, Hu and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abel S., Luckheide N., Westendorf A. M., Geffers R., Roers A., Muller W., et al. (2012). Strong impact of CD4+ Foxp3+ regulatory T cells and limited effect of T cell-derived IL-10 on pathogen clearance during Plasmodium yoelii infection. J. Immunol. 188, 5467–5477. 10.4049/jimmunol.1102223 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

