Bifunctional, Copper-Doped, Mesoporous Silica Nanosphere-Modified, Bioceramic Scaffolds for Bone Tumor Therapy
- PMID: 33363114
- PMCID: PMC7755992
- DOI: 10.3389/fchem.2020.610232
Bifunctional, Copper-Doped, Mesoporous Silica Nanosphere-Modified, Bioceramic Scaffolds for Bone Tumor Therapy
Abstract
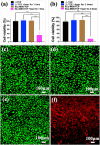
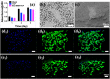
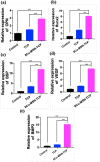
In the traditional surgical intervention procedure, residual tumor cells may potentially cause tumor recurrence. In addition, large bone defects caused by surgery are difficult to self-repair. Thus, it is necessary to design a bioactive scaffold that can not only kill residual tumor cells but also promote bone defect regeneration simultaneously. Here, we successfully developed Cu-containing mesoporous silica nanosphere-modified β-tricalcium phosphate (Cu-MSN-TCP) scaffolds, with uniform and dense nanolayers with spherical morphology via 3D printing and spin coating. The scaffolds exhibited coating time- and laser power density-dependent photothermal performance, which favored the effective killing of tumor cells under near-infrared laser irradiation. Furthermore, the prepared scaffolds favored the proliferation and attachment of rabbit bone marrow-derived mesenchymal stem cells and stimulated the gene expression of osteogenic markers. Overall, Cu-MSN-TCP scaffolds can be considered for complete eradication of residual bone tumor cells and simultaneous healing of large bone defects, which may provide a novel and effective strategy for bone tumor therapy. In the future, such Cu-MSN-TCP scaffolds may function as carriers of anti-cancer drugs or immune checkpoint inhibitors in chemo-/photothermal or immune-/photothermal therapy of bone tumors, favoring for effective treatment.
Keywords: 3D printing; Cu-containing mesoporous silica nanospheres; bifunctional scaffolds; photothermal tumor therapy; tissue regeneration.
Copyright © 2020 Ma, Ma, Chen, Li, Liu, Ma, Mao, Yang, Ma and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
3D-printed scaffolds with bioactive elements-induced photothermal effect for bone tumor therapy.Acta Biomater. 2018 Jun;73:531-546. doi: 10.1016/j.actbio.2018.04.014. Epub 2018 Apr 13. Acta Biomater. 2018. PMID: 29656075
-
3D printing of metal-organic framework nanosheets-structured scaffolds with tumor therapy and bone construction.Biofabrication. 2020 Jan 31;12(2):025005. doi: 10.1088/1758-5090/ab5ae3. Biofabrication. 2020. PMID: 31756727
-
Mesoporous bioactive glass nanolayer-functionalized 3D-printed scaffolds for accelerating osteogenesis and angiogenesis.Nanoscale. 2015 Dec 7;7(45):19207-21. doi: 10.1039/c5nr05421d. Epub 2015 Nov 3. Nanoscale. 2015. PMID: 26525451
-
Two Hawks with One Arrow: A Review on Bifunctional Scaffolds for Photothermal Therapy and Bone Regeneration.Nanomaterials (Basel). 2023 Jan 29;13(3):551. doi: 10.3390/nano13030551. Nanomaterials (Basel). 2023. PMID: 36770512 Free PMC article. Review.
-
3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy.Acta Biomater. 2018 Oct 1;79:37-59. doi: 10.1016/j.actbio.2018.08.026. Epub 2018 Aug 28. Acta Biomater. 2018. PMID: 30165201 Review.
Cited by
-
Application of advanced biomaterials in photothermal therapy for malignant bone tumors.Biomater Res. 2023 Nov 15;27(1):116. doi: 10.1186/s40824-023-00453-z. Biomater Res. 2023. PMID: 37968707 Free PMC article. Review.
-
Bifunctional scaffolds for tumor therapy and bone regeneration: Synergistic effect and interplay between therapeutic agents and scaffold materials.Mater Today Bio. 2022 Jun 9;15:100318. doi: 10.1016/j.mtbio.2022.100318. eCollection 2022 Jun. Mater Today Bio. 2022. PMID: 35734197 Free PMC article. Review.
-
Inorganic Nanoparticles in Bone Healing Applications.Pharmaceutics. 2022 Mar 31;14(4):770. doi: 10.3390/pharmaceutics14040770. Pharmaceutics. 2022. PMID: 35456604 Free PMC article. Review.
-
Progress of Phototherapy Applications in the Treatment of Bone Cancer.Int J Mol Sci. 2021 Oct 21;22(21):11354. doi: 10.3390/ijms222111354. Int J Mol Sci. 2021. PMID: 34768789 Free PMC article. Review.
-
Ion Doped Hollow Silica Nanoparticles as Promising Oligonucleotide Delivery Systems to Mesenchymal Stem Cells.Int J Nanomedicine. 2024 Sep 20;19:9741-9755. doi: 10.2147/IJN.S461167. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39329032 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

