Building Scaffolds for Tubular Tissue Engineering
- PMID: 33363127
- PMCID: PMC7758256
- DOI: 10.3389/fbioe.2020.589960
Building Scaffolds for Tubular Tissue Engineering
Abstract
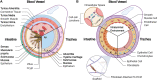
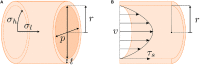
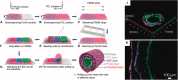
Hollow organs and tissue systems drive various functions in the body. Many of these hollow or tubular systems, such as vasculature, the intestines, and the trachea, are common targets for tissue engineering, given their relevance to numerous diseases and body functions. As the field of tissue engineering has developed, numerous benchtop models have been produced as platforms for basic science and drug testing. Production of tubular scaffolds for different tissue engineering applications possesses many commonalities, such as the necessity for producing an intact tubular opening and for formation of semi-permeable epithelia or endothelia. As such, the field has converged on a series of manufacturing techniques for producing these structures. In this review, we discuss some of the most common tissue engineered applications within the context of tubular tissues and the methods by which these structures can be produced. We provide an overview of the general structure and anatomy for these tissue systems along with a series of general design criteria for tubular tissue engineering. We categorize methods for manufacturing tubular scaffolds as follows: casting, electrospinning, rolling, 3D printing, and decellularization. We discuss state-of-the-art models within the context of vascular, intestinal, and tracheal tissue engineering. Finally, we conclude with a discussion of the future for these fields.
Keywords: 3D printing; biomaterials; decellularization; electrospinning; intestine; lumen; trachea; vascular.
Copyright © 2020 Boys, Barron, Tilev and Owens.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

