Real-Time Molecular Monitoring in Acute Myeloid Leukemia With Circulating Tumor DNA
- PMID: 33363162
- PMCID: PMC7759522
- DOI: 10.3389/fcell.2020.604391
Real-Time Molecular Monitoring in Acute Myeloid Leukemia With Circulating Tumor DNA
Abstract
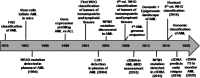
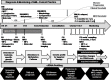
The clonal evolution of acute myeloid leukemia (AML), an oligoclonal hematological malignancy, is driven by a plethora of cytogenetic abnormalities, gene mutations, abnormal epigenetic patterns, and aberrant gene expressions. These alterations in the leukemic blasts promote clinically diverse manifestations with common characteristics of high relapse and drug resistance. Defining and real-time monitoring of a personalized panel of these predictive genetic biomarkers is rapidly being adapted in clinical setting for diagnostic, prognostic, and therapeutic decision-making in AML. A major challenge remains the frequency of invasive biopsy procedures that can be routinely performed for monitoring of AML disease progression. Moreover, a single-site biopsy is not representative of the tumor heterogeneity as it is spatially and temporally constrained and necessitates the understanding of longitudinal and spatial subclonal dynamics in AML. Hematopoietic cells are a major contributor to plasma cell-free DNA, which also contain leukemia-specific aberrations as the circulating tumor-derived DNA (ctDNA) fraction. Plasma cell-free DNA analysis holds immense potential as a minimally invasive tool for genomic profiling at diagnosis as well as clonal evolution during AML disease progression. With the technological advances and increasing sensitivity for detection of ctDNA, both genetic and epigenetic aberrations can be qualitatively and quantitatively evaluated. However, challenges remain in validating the utility of liquid biopsy tools in clinics, and universal recommendations are still awaited towards reliable diagnostics and prognostics. Here, we provide an overview on the scope of ctDNA analyses for prognosis, assessment of response to treatment and measurable residual disease, prediction of disease relapse, development of acquired resistance and beyond in AML.
Keywords: acute myeloid leukemia; cfDNA; circulating tumor DNA; ctDNA; genomic profiling; hematological malignancies; measurable residual disease; plasma cell free DNA.
Copyright © 2020 Thakral, Gupta, Sahoo, Verma, Kumar and Vashishtha.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Advancing Leukemia Management Through Liquid Biopsy: Insights into Biomarkers and Clinical Utility.Cancers (Basel). 2025 Apr 25;17(9):1438. doi: 10.3390/cancers17091438. Cancers (Basel). 2025. PMID: 40361366 Free PMC article. Review.
-
A comprehensive review of genetic alterations and molecular targeted therapies for the implementation of personalized medicine in acute myeloid leukemia.J Mol Med (Berl). 2020 Aug;98(8):1069-1091. doi: 10.1007/s00109-020-01944-5. Epub 2020 Jul 3. J Mol Med (Berl). 2020. PMID: 32620999 Review.
-
Cell-free DNA for genomic profiling and minimal residual disease monitoring in Myeloma- are we there yet?Am J Blood Res. 2020 Jun 15;10(3):26-45. eCollection 2020. Am J Blood Res. 2020. PMID: 32685257 Free PMC article. Review.
-
Cell-free DNA - Minimally invasive marker of hematological malignancies.Eur J Haematol. 2017 Oct;99(4):291-299. doi: 10.1111/ejh.12925. Epub 2017 Aug 3. Eur J Haematol. 2017. PMID: 28692178 Review.
-
Dynamic Treatment Stratification Using ctDNA.Recent Results Cancer Res. 2020;215:263-273. doi: 10.1007/978-3-030-26439-0_14. Recent Results Cancer Res. 2020. PMID: 31605234 Review.
Cited by
-
Functions and clinical significance of circular RNAs in acute myeloid leukemia.Front Pharmacol. 2022 Nov 24;13:1010579. doi: 10.3389/fphar.2022.1010579. eCollection 2022. Front Pharmacol. 2022. PMID: 36506538 Free PMC article. Review.
-
Impact of Long-Term Plasma Storage on Cell-Free DNA Epigenetic Biomarker Studies.Biomolecules. 2025 Jun 25;15(7):927. doi: 10.3390/biom15070927. Biomolecules. 2025. PMID: 40723799 Free PMC article.
-
Integrated analysis of circulating cell free nucleic acids for cancer genotyping and immune phenotyping of tumor microenvironment.Front Genet. 2023 Apr 6;14:1138625. doi: 10.3389/fgene.2023.1138625. eCollection 2023. Front Genet. 2023. PMID: 37091783 Free PMC article. Review.
-
Liquid biopsies and minimal residual disease in myeloid malignancies.Front Oncol. 2023 May 5;13:1164017. doi: 10.3389/fonc.2023.1164017. eCollection 2023. Front Oncol. 2023. PMID: 37213280 Free PMC article. Review.
-
Impact of Preanalytical and Analytical Methods on Cell-Free DNA Diagnostics.Front Cell Dev Biol. 2021 Sep 6;9:686149. doi: 10.3389/fcell.2021.686149. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34552921 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

