Cyclic Stretch Induces Vascular Smooth Muscle Cells to Secrete Connective Tissue Growth Factor and Promote Endothelial Progenitor Cell Differentiation and Angiogenesis
- PMID: 33363166
- PMCID: PMC7755638
- DOI: 10.3389/fcell.2020.606989
Cyclic Stretch Induces Vascular Smooth Muscle Cells to Secrete Connective Tissue Growth Factor and Promote Endothelial Progenitor Cell Differentiation and Angiogenesis
Abstract
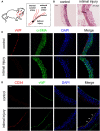
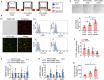
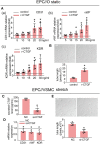
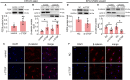
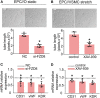
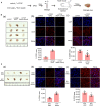
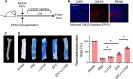
Endothelial progenitor cells (EPCs) play a vital role in endothelial repair following vascular injury by maintaining the integrity of endothelium. As EPCs home to endothelial injury sites, they may communicate with exposed vascular smooth muscle cells (VSMCs), which are subjected to cyclic stretch generated by blood flow. In this study, the synergistic effect of cyclic stretch and communication with neighboring VSMCs on EPC function during vascular repair was investigated. In vivo study revealed that EPCs adhered to the injury site and were contacted to VSMCs in the Sprague-Dawley (SD) rat carotid artery injury model. In vitro, EPCs were cocultured with VSMCs, which were exposed to cyclic stretch at a magnitude of 5% (which mimics physiological stretch) and a constant frequency of 1.25 Hz for 12 h. The results indicated that stretched VSMCs modulated EPC differentiation into mature endothelial cells (ECs) and promoted angiogenesis. Meanwhile, cyclic stretch upregulated the mRNA expression and secretion level of connective tissue growth factor (CTGF) in VSMCs. Recombinant CTGF (r-CTGF) treatment promoted endothelial differentiation of EPCs and angiogenesis, and increased their protein levels of FZD8 and β-catenin. CTGF knockdown in VSMCs inhibited cyclic stretch-induced EPC differentiation into ECs and attenuated EPC tube formation via modulation of the FZD8/β-catenin signaling pathway. FZD8 knockdown repressed endothelial differentiation of EPCs and their angiogenic activity. Wnt signaling inhibitor decreased the endothelial differentiation and angiogenetic ability of EPCs cocultured with stretched VSMCs. Consistently, an in vivo Matrigel plug assay demonstrated that r-CTGF-treated EPCs exhibited enhanced angiogenesis; similarly, stretched VSMCs also induced cocultured EPC differentiation toward ECs. In a rat vascular injury model, r-CTGF improved EPC reendothelialization capacity. The present results indicate that cyclic stretch induces VSMC-derived CTGF secretion, which, in turn, activates FZD8 and β-catenin to promote both differentiation of cocultured EPCs into the EC lineage and angiogenesis, suggesting that CTGF acts as a key intercellular mediator and a potential therapeutic target for vascular repair.
Keywords: angiogenesis; connective tissue growth factor; cyclic stretch; differentiation; endothelial progenitor cells; vascular smooth muscle cells.
Copyright © 2020 Yan, Wang, Fan, Bao, Li, Yao, Huo, Jiang, Qi and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Blomme B., Deroanne C., Hulin A., Lambert C., Defraigne J. O., Nusgens B., et al. (2019). Mechanical strain induces a pro-fibrotic phenotype in human mitral valvular interstitial cells through RhoC/ROCK/MRTF-A and Erk1/2 signaling pathways. J. Mol. Cell Cardiol. 135, 149–159. 10.1016/j.yjmcc.2019.08.008 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

