Zoonotic Tick-Borne Pathogens in Temperate and Cold Regions of Europe-A Review on the Prevalence in Domestic Animals
- PMID: 33363242
- PMCID: PMC7758354
- DOI: 10.3389/fvets.2020.604910
Zoonotic Tick-Borne Pathogens in Temperate and Cold Regions of Europe-A Review on the Prevalence in Domestic Animals
Abstract
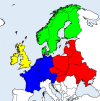
Ticks transmit a variety of pathogens affecting both human and animal health. In temperate and cold regions of Europe (Western, Central, Eastern, and Northern Europe), the most relevant zoonotic tick-borne pathogens are tick-borne encephalitis virus (TBEV), Borrelia spp. and Anaplasma phagocytophilum. More rarely, Rickettsia spp., Neoehrlichia mikurensis, and zoonotic Babesia spp. are identified as a cause of human disease. Domestic animals may also be clinically affected by these pathogens, and, furthermore, can be regarded as sentinel hosts for their occurrence in a certain area, or even play a role as reservoirs or amplifying hosts. For example, viraemic ruminants may transmit TBEV to humans via raw milk products. This review summarizes the role of domestic animals, including ruminants, horses, dogs, and cats, in the ecology of TBEV, Borrelia spp., A. phagocytophilum, Rickettsia spp., N. mikurensis, and zoonotic Babesia species. It gives an overview on the (sero-)prevalence of these infectious agents in domestic animals in temperate/cold regions of Europe, based on 148 individual prevalence studies. Meta-analyses of seroprevalence in asymptomatic animals estimated an overall seroprevalence of 2.7% for TBEV, 12.9% for Borrelia burgdorferi sensu lato (s.l.), 16.2% for A. phagocytophilum and 7.4% for Babesia divergens, with a high level of heterogeneity. Subgroup analyses with regard to animal species, diagnostic test, geographical region and decade of sampling were mostly non-significant, with the exception of significantly lower B. burgdorferi s.l. seroprevalences in dogs than in horses and cattle. More surveillance studies employing highly sensitive and specific test methods and including hitherto non-investigated regions are needed to determine if and how global changes in terms of climate, land use, agricultural practices and human behavior impact the frequency of zoonotic tick-borne pathogens in domestic animals.
Keywords: Anaplasma; Babesia; Borrelia; Neoehrlichia mikurensis; Rickettsia; tick-borne diseases; tick-borne encephalitis; vector-borne diseases.
Copyright © 2020 Springer, Glass, Topp and Strube.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- WHO/FAO . Joint FAO/WHO Expert Committee on Zoonoses [Meeting Held in Geneva from 6 to 12 December 1966]: Third Report. World Health Organization, Geneva: (1967).
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

