Barriers and Opportunities for Implementation of Outcome-Based Spread Payments for High-Cost, One-Shot Curative Therapies
- PMID: 33363468
- PMCID: PMC7753155
- DOI: 10.3389/fphar.2020.594446
Barriers and Opportunities for Implementation of Outcome-Based Spread Payments for High-Cost, One-Shot Curative Therapies
Abstract
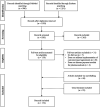
Background: The challenging market access of high-cost one-time curative therapies has inspired the development of alternative reimbursement structures, such as outcome-based spread payments, to mitigate their unaffordability and answer remaining uncertainties. This study aimed to provide a broad overview of barriers and possible opportunities for the practical implementation of outcome-based spread payments for the reimbursement of one-shot therapies in European healthcare systems. Methods: A systematic literature review was performed investigating published literature and publicly available documents to identify barriers and implementation opportunities for both spreading payments and for implementing outcome-based agreements. Data was analyzed via qualitative content analysis by extracting data with a reporting template. Results: A total of 1,503 publications were screened and 174 were included. Main identified barriers for the implementation of spread payments are reaching an agreement on financial terms while considering 12-months budget cycles and the possible violation of corresponding international accounting rules. Furthermore, outcome correction of payments is currently hindered by the need for additional data collection, the lack of clear governance structures and the resulting administrative burden and cost. The use of spread payments adjusted by population- or individual-level data collected within automated registries and overseen by a governance committee and external advisory board may alleviate several barriers and may support the reimbursement of highly innovative therapies. Conclusion: High-cost advanced therapy medicinal products pose a substantial affordability challenge on healthcare systems worldwide. Outcome-based spread payments may mitigate the initial budget impact and alleviate existing uncertainties; however, their effective implementation still faces several barriers and will be facilitated by realizing the required organizational changes.
Keywords: advanced therapy medicinal product; affordability; annuity; curative therapy; managed entry agreement; outcome-based agreement; pay-for-performance; spread payment.
Copyright © 2020 Michelsen, Nachi, Van Dyck, Simoens and Huys.
Conflict of interest statement
SS has provided advice to Novartis about the design of a managed entry agreement for an advanced therapy medicinal product. VW has led a Pfizer-sponsored Belgian national Round Table gathering business and societal perspectives on high-priced gene therapies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Alliance for Regenerative Medicine (2019). Getting ready: recommendations for timely access to advanced therapy medicinal products (ATMPs) in Europe.
Publication types
LinkOut - more resources
Full Text Sources