Amniotic Membrane Extract Protects Islets From Serum-Deprivation Induced Impairments and Improves Islet Transplantation Outcome
- PMID: 33363516
- PMCID: PMC7753361
- DOI: 10.3389/fendo.2020.587450
Amniotic Membrane Extract Protects Islets From Serum-Deprivation Induced Impairments and Improves Islet Transplantation Outcome
Abstract
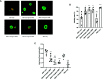
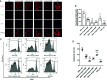
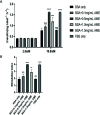
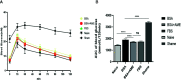
Islet culture prior to transplantation is a standard practice in many transplantation centers. Nevertheless, the abundant islet mass loss and function impairment during this serum-deprivation culture period restrain the success of islet transplantation. In the present study, we used a natural biomaterial derived product, amniotic membrane extract (AME), as medium supplementation of islet pretransplant cultivation to investigate its protective effect on islet survival and function and its underlying mechanisms, as well as the engraftment outcome of islets following AME treatment. Results showed that AME supplementation improved islet viability and function, and decreased islet apoptosis and islet loss during serum-deprived culture. This was associated with the increased phosphorylation of PI3K/Akt and MAPK/ERK signaling pathway. Moreover, transplantation of serum-deprivation stressed islets that were pre-treated with AME into diabetic mice revealed better blood glucose control and improved islet graft survival. In conclusion, AME could improve islet survival and function in vivo and in vitro, and was at least partially through increasing phosphorylation of PI3K/Akt and MAPK/ERK signaling pathway.
Keywords: amniotic membrane extract; apoptosis; islet transplantation; serum-deprivation; type 1 diabetes.
Copyright © 2020 Yang, Li, Zhang, Sun, Guo, Lin, Li and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Glial cell line-derived neurotrophic factor enhances human islet posttransplantation survival.Transplantation. 2011 Oct 15;92(7):745-51. doi: 10.1097/TP.0b013e31822bc95a. Transplantation. 2011. PMID: 21869742 Free PMC article.
-
Engraftment Site and Effectiveness of the Pan-Caspase Inhibitor F573 to Improve Engraftment in Mouse and Human Islet Transplantation in Mice.Transplantation. 2017 Oct;101(10):2321-2329. doi: 10.1097/TP.0000000000001638. Transplantation. 2017. PMID: 28072753
-
A preconditioning regimen with a PKCɛ activator improves islet graft function in a mouse transplant model.Cell Transplant. 2014;23(7):913-9. doi: 10.3727/096368913X665567. Epub 2013 Apr 2. Cell Transplant. 2014. PMID: 23562311
-
Mesenchymal stromal cell secretory factors induce sustained improvements in islet function pre- and post-transplantation.Cytotherapy. 2018 Dec;20(12):1427-1436. doi: 10.1016/j.jcyt.2018.07.007. Epub 2018 Oct 27. Cytotherapy. 2018. PMID: 30377040
-
Red Ginseng Administration Before Islet Isolation Attenuates Apoptosis and Improves Islet Function and Transplant Outcome in a Syngeneic Mouse Marginal Islet Mass Model.Transplant Proc. 2016 May;48(4):1258-65. doi: 10.1016/j.transproceed.2016.01.025. Transplant Proc. 2016. PMID: 27320599
Cited by
-
The Influence of Microenvironment on Survival of Intraportal Transplanted Islets.Front Immunol. 2022 Mar 28;13:849580. doi: 10.3389/fimmu.2022.849580. eCollection 2022. Front Immunol. 2022. PMID: 35418988 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

