Human Serum Albumin Binds Streptolysin O (SLO) Toxin Produced by Group A Streptococcus and Inhibits Its Cytotoxic and Hemolytic Effects
- PMID: 33363530
- PMCID: PMC7752801
- DOI: 10.3389/fimmu.2020.507092
Human Serum Albumin Binds Streptolysin O (SLO) Toxin Produced by Group A Streptococcus and Inhibits Its Cytotoxic and Hemolytic Effects
Abstract
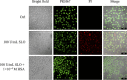
The pathogenicity of group A Streptococcus (GAS) is mediated by direct bacterial invasivity and toxin-associated damage. Among the extracellular products, the exotoxin streptolysin O (SLO) is produced by almost all GAS strains. SLO is a pore forming toxin (PFT) hemolitically active and extremely toxic in vivo. Recent evidence suggests that human serum albumin (HSA), the most abundant protein in plasma, is a player in the innate immunity "orchestra." We previously demonstrated that HSA acts as a physiological buffer, partially neutralizing Clostridioides difficile toxins that reach the bloodstream after being produced in the colon. Here, we report the in vitro and ex vivo capability of HSA to neutralize the cytotoxic and hemolytic effects of SLO. HSA binds SLO with high affinity at a non-conventional site located in domain II, which was previously reported to interact also with C. difficile toxins. HSA:SLO recognition protects HEp-2 and A549 cells from cytotoxic effects and cell membrane permeabilization induced by SLO. Moreover, HSA inhibits the SLO-dependent hemolytic effect in red blood cells isolated from healthy human donors. The recognition of SLO by HSA may have a significant protective role in human serum and sustains the emerging hypothesis that HSA is an important constituent of the innate immunity system.
Keywords: Streptococcus pyogenes; human serum albumin; red blood cells; streptolysin O; toxin.
Copyright © 2020 Vita, De Simone, Leboffe, Montagnani, Mariotti, Di Bella, Luzzati, Gori, Ascenzi and di Masi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- BWaT EW. Lesions in the mouse produced by streptolysins O and S. J Pathol (1940) 51:5. 10.1002/path.1700510108 - DOI

