Single-Stranded Oligonucleotide-Mediated Inhibition of Respiratory Syncytial Virus Infection
- PMID: 33363532
- PMCID: PMC7752805
- DOI: 10.3389/fimmu.2020.580547
Single-Stranded Oligonucleotide-Mediated Inhibition of Respiratory Syncytial Virus Infection
Abstract
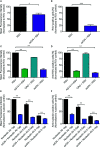
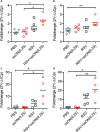
Respiratory syncytial virus (RSV) is the leading cause of acute lower respiratory tract infections in young children. Currently, there is no RSV vaccine or universally accessible antiviral treatment available. Addressing the urgent need for new antiviral agents, we have investigated the capacity of a non-coding single-stranded oligonucleotide (ssON) to inhibit RSV infection. By utilizing a GFP-expressing RSV, we demonstrate that the ssON significantly reduced the proportion of RSV infected A549 cells (lung epithelial cells). Furthermore, we show that ssON's antiviral activity was length dependent and that both RNA and DNA of this class of oligonucleotides have antiviral activity. We reveal that ssON inhibited RSV infection by competing with the virus for binding to the cellular receptor nucleolin in vitro. Additionally, using a recombinant RSV that expresses luciferase we show that ssON effectively blocked RSV infection in mice. Treatment with ssON in vivo resulted in the upregulation of RSV-induced interferon stimulated genes (ISGs) such as Stat1, Stat2, Cxcl10, and Ccl2. This study highlights the possibility of using oligonucleotides as therapeutic agents against RSV infection. We demonstrate that the mechanism of action of ssON is the inhibition of viral entry in vitro, likely through the binding of the receptor, nucleolin and that ssON treatment against RSV infection in vivo additionally results in the upregulation of ISGs.
Keywords: ISGs; antiviral; nucleolin; oligonucleotides; respiratory syncytial virus (RSV); single-stranded oligonucleotides; ssON.
Copyright © 2020 Pålsson, Dondalska, Bergenstråhle, Rolfes, Björk, Sedano, Power, Rameix-Welti, Lundeberg, Wahren-Herlenius, Mastrangelo, Eleouet, Le Goffic, Galloux and Spetz.
Conflict of interest statement
AD and A-LS are shareholders of TIRmed Pharma in possession of intellectual properties related to ssON. A-LS is CEO of TIRmed Pharma. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Collins PL, Fearns R, Graham BS. “Respiratory Syncytial Virus: Virology, Reverse Genetics, and Pathogenesis of Disease”. In: Anderson LJ, Graham BS, editors. Challenges and Opportunities for Respiratory Syncytial Virus Vaccines. Berlin, Heidelberg: Springer Berlin Heidelberg; (2013). p. 3–38. 10.1007/978-3-642-38919-1_1 - DOI - PMC - PubMed
-
- Shi T, Ooi Y, Zaw EM, Utjesanovic N, Campbell H, Cunningham S, et al. Association Between Respiratory Syncytial Virus-Associated Acute Lower Respiratory Infection in Early Life and Recurrent Wheeze and Asthma in Later Childhood. J Infect Dis (2019) 2(Issue Supplement 7):jiz311. 10.1093/infdis/jiz311 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

