Methods to Detect MHC-Specific IgE in Mice and Men
- PMID: 33363535
- PMCID: PMC7753192
- DOI: 10.3389/fimmu.2020.586856
Methods to Detect MHC-Specific IgE in Mice and Men
Abstract
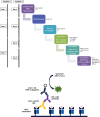
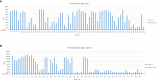
Humoral immunity is a major barrier limiting long-term outcome after organ transplantation. Especially, the production of antibodies directed against donor HLA/MHC antigens (i.e. donor-specific antibodies (DSA)) leading to antibody-mediated rejection (ABMR) is considered to be a major factor negatively affecting allograft survival. DSAs of the IgG isotype are routinely measured in transplant patients. However, not all patients diagnosed with IgG-DSA develop ABMR events. Therefore, research in better understanding the mechanisms of ABMR is of great importance. We recently demonstrated the production of MHC-specific IgE upon allograft rejection in mice and in transplant patients. IgE is classically connected with allergy and is known to be important for the humoral defense against helminths and worms. However, its role in autoimmune diseases and cancer has been reported recently as well. The concentration of IgE in blood is extremely low compared to other antibody isotypes. Therefore, detection of MHC-specific IgE from serum requires methods of high sensitivity. Since MHC-specific IgG-typically present at much higher serum levels-develops as well, high specificity is also required of IgE detection methods. In the murine model we developed an enzyme linked immunosorbent assay (ELISA) using MHC monomers for measurement of MHC-specific IgE, allowing us to distinguish between specificities of antibodies against different class I and class II antigens. For measurement of functional activity of MHC-specific IgE in vitro, a release assay using a rat basophil cell line (RBL-2H3) was established. For functional analysis of MHC-specific IgE in vivo, a cutaneous hypersensitivity reaction assay was adapted for this purpose using MHC monomers. Humanized RBL-2H3 cells transfected with cDNA coding for the human-high affinity IgE receptor were used for functionality measurement of donor-specific IgE in sensitized transplant patients. For detection of HLA-specific IgE, a bead assay was adapted, using beads expressing single HLA antigens. The aim of this publication is to demonstrate currently established methods for the detection and characterization of MHC-specific IgE in the murine and human setting.
Keywords: HLA; IgE; MHC; antibody-mediated rejection; donor specific antibodies; transplantation.
Copyright © 2020 Weijler, Mucha, Farkas, Baranyi, Pilat, Cho, Muckenhuber, Hopf, Wahrmann, Linhart, Valenta and Wekerle.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

