Damage-Associated Molecular Patterns in Myocardial Infarction and Heart Transplantation: The Road to Translational Success
- PMID: 33363540
- PMCID: PMC7752942
- DOI: 10.3389/fimmu.2020.599511
Damage-Associated Molecular Patterns in Myocardial Infarction and Heart Transplantation: The Road to Translational Success
Abstract
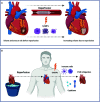
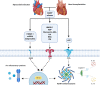
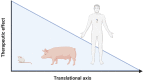
In the setting of myocardial infarction (MI), ischemia reperfusion injury (IRI) occurs due to occlusion (ischemia) and subsequent re-establishment of blood flow (reperfusion) of a coronary artery. A similar phenomenon is observed in heart transplantation (HTx) when, after cold storage, the donor heart is connected to the recipient's circulation. Although reperfusion is essential for the survival of cardiomyocytes, it paradoxically leads to additional myocardial damage in experimental MI and HTx models. Damage (or danger)-associated molecular patterns (DAMPs) are endogenous molecules released after cellular damage or stress such as myocardial IRI. DAMPs activate pattern recognition receptors (PRRs), and set in motion a complex signaling cascade resulting in the release of cytokines and a profound inflammatory reaction. This inflammatory response is thought to function as a double-edged sword. Although it enables removal of cell debris and promotes wound healing, DAMP mediated signalling can also exacerbate the inflammatory state in a disproportional matter, thereby leading to additional tissue damage. Upon MI, this leads to expansion of the infarcted area and deterioration of cardiac function in preclinical models. Eventually this culminates in adverse myocardial remodeling; a process that leads to increased myocardial fibrosis, gradual further loss of cardiomyocytes, left ventricular dilation and heart failure. Upon HTx, DAMPs aggravate ischemic damage, which results in more pronounced reperfusion injury that impacts cardiac function and increases the occurrence of primary graft dysfunction and graft rejection via cytokine release, cardiac edema, enhanced myocardial/endothelial damage and allograft fibrosis. Therapies targeting DAMPs or PRRs have predominantly been investigated in experimental models and are potentially cardioprotective. To date, however, none of these interventions have reached the clinical arena. In this review we summarize the current evidence of involvement of DAMPs and PRRs in the inflammatory response after MI and HTx. Furthermore, we will discuss various current therapeutic approaches targeting this complex interplay and provide possible reasons why clinical translation still fails.
Keywords: damage-associated molecular patterns; heart transplantation; innate immunity; ischemia reperfusion injury; myocardial infarction; pattern recognition receptors; sterile inflammation.
Copyright © 2020 Silvis, Kaffka genaamd Dengler, Odille, Mishra, van der Kaaij, Doevendans, Sluijter, de Kleijn, de Jager, Bosch and van Hout.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Lund LH, Khush KK, Cherikh WS, Goldfarb S, Kucheryavaya AY, Levvey BJ, et al. The Registry of the International Society for Heart and LungTransplantation: Thirty-fourth Adult Heart Transplantation Report—2017; Focus Theme: Allograftischemic time. J Hear Lung Transplant (2017) 36:1037–46. 10.1016/j.healun.2017.07.019 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

