CD86 Is a Selective CD28 Ligand Supporting FoxP3+ Regulatory T Cell Homeostasis in the Presence of High Levels of CTLA-4
- PMID: 33363541
- PMCID: PMC7753196
- DOI: 10.3389/fimmu.2020.600000
CD86 Is a Selective CD28 Ligand Supporting FoxP3+ Regulatory T Cell Homeostasis in the Presence of High Levels of CTLA-4
Abstract
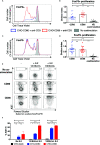
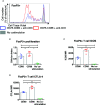
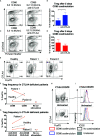
CD80 and CD86 are expressed on antigen presenting cells and are required to engage their shared receptor, CD28, for the costimulation of CD4 T cells. It is unclear why two stimulatory ligands with overlapping roles have evolved. CD80 and CD86 also bind the regulatory molecule CTLA-4. We explored the role of CD80 and CD86 in the homeostasis and proliferation of CD4+FoxP3+ regulatory T cells (Treg), which constitutively express high levels of CTLA-4 yet are critically dependent upon CD28 signals. We observed that CD86 was the dominant ligand for Treg proliferation, survival, and maintenance of a regulatory phenotype, with higher expression of CTLA-4, ICOS, and OX40. We also explored whether CD80-CD28 interactions were specifically compromised by CTLA-4 and found that antibody blockade, clinical deficiency of CTLA-4 and CRISPR-Cas9 deletion of CTLA-4 all improved Treg survival following CD80 stimulation. Taken together, our data suggest that CD86 is the dominant costimulatory ligand for Treg homeostasis, despite its lower affinity for CD28, because CD80-CD28 interactions are selectively impaired by the high levels of CTLA-4. These data suggest a cell intrinsic role for CTLA-4 in regulating CD28 costimulation by direct competition for CD80, and indicate that that CD80 and CD86 have discrete roles in CD28 costimulation of CD4 T cells in the presence of high levels of CTLA-4.
Keywords: CD28; CD80; CD86; CTLA-4; costimulation; homeostasis; regulatory T cells.
Copyright © 2020 Halliday, Williams, Kennedy, Waters, Pesenacker, Soskic, Hinze, Hou, Rowshanravan, Janman, Walker and Sansom.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

