An overview of the tumor microenvironment, from cells to complex networks (Review)
- PMID: 33363607
- PMCID: PMC7725019
- DOI: 10.3892/etm.2020.9528
An overview of the tumor microenvironment, from cells to complex networks (Review)
Abstract
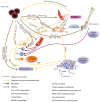
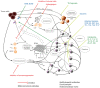
For a long period, cancer has been believed to be a gene disease, in which oncogenic and suppressor mutations accumulate gradually, finally leading to the malignant transformation of cells. This vision has changed in the last few years, the involvement of the tumor microenvironment, the non-malignant part of the tumors, as an important contributor to the malignant growth being now largely recognized. There is a consensus according to which the understanding of the tumor microenvironment is important as a means to develop new approaches in the therapy of cancer. In this context, the present study is a review of the different types of non-malignant cells that can be found in tumors, with their pro or antitumoral actions, presence in tumors and therapeutic targeting. These cells establish complex relations between them, through cytokines, exosomes, cell adhesion, co-stimulation and co-inhibition; these relations will also be examined in the present work.
Keywords: biogenesis; immunity; microenvironment; network.
Copyright: © Farc et al.
Figures


References
-
- Murphy K, Weaver C, Janeway C. Janeway's immunobiology. 9th edition. New York, Garland Science, 2017.
Publication types
LinkOut - more resources
Full Text Sources
