Derivation of a nuclear heterogeneity image index to grade DCIS
- PMID: 33363702
- PMCID: PMC7744935
- DOI: 10.1016/j.csbj.2020.11.040
Derivation of a nuclear heterogeneity image index to grade DCIS
Abstract
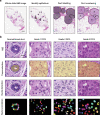
Abnormalities in cell nuclear morphology are a hallmark of cancer. Histological assessment of cell nuclear morphology is frequently used by pathologists to grade ductal carcinoma in situ (DCIS). Objective methods that allow standardization and reproducibility of cell nuclear morphology assessment have potential to improve the criteria needed to predict DCIS progression and recurrence. Aggressive cancers are highly heterogeneous. We asked whether cell nuclear morphology heterogeneity could be incorporated into a metric to classify DCIS. We developed a nuclear heterogeneity image index to objectively, and quantitatively grade DCIS. A whole-tissue cell nuclear morphological analysis, that classified tumors by the worst ten percent in a duct-by-duct manner, identified nuclear size ranges associated with each DCIS grade. Digital image analysis further revealed increasing heterogeneity within ducts or between ducts in tissues of worsening DCIS grade. The findings illustrate how digital image analysis comprises a supplemental tool for pathologists to objectively classify DCIS and in the future, may provide a method to predict patient outcome through analysis of nuclear heterogeneity.
Keywords: Breast cancer; Heterogeneity; Image analysis; Nuclear morphology; Pathology.
© 2020 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Lester S.C. Protocol for the examination of specimens from patients with ductal carcinoma in situ of the breast. Arch Pathol Lab Med. 2009;133(1):15–25. - PubMed
-
- Badve S., A'hern R.P., Ward A.M., Millis R.R., Pinder S.E., Ellis I.O., Gusterson B.A., Sloane J.P. Prediction of local recurrence of ductal carcinoma in situ of the breast using five histological classifications: a comparative study with long follow-up. Hum Pathol. 1998;29(9):915–923. doi: 10.1016/S0046-8177(98)90196-4. - DOI - PubMed
