New biodegradable nanoparticles-in-nanofibers based membranes for guided periodontal tissue and bone regeneration with enhanced antibacterial activity
- PMID: 33364045
- PMCID: PMC7753955
- DOI: 10.1016/j.jare.2020.06.014
New biodegradable nanoparticles-in-nanofibers based membranes for guided periodontal tissue and bone regeneration with enhanced antibacterial activity
Abstract
Introduction: Guided tissue regeneration (GTR) and guided bone regeneration (GBR) are commonly used surgical procedures for the repair of damaged periodontal tissues. These procedures include the use of a membrane as barrier to prevent soft tissue ingrowth and to create space for slowly regenerating periodontium and bone. Recent approaches involve the use of membranes/scaffolds based on resorbable materials. These materials provide the advantage of dissolving by time without the need of surgical intervention to remove the scaffolds.
Objectives: This study aimed at preparing a new series of nanofibrous scaffolds for GTR/GBR applications with enhanced mechanical properties, cell adhesion, biocompatibility and antibacterial properties.
Methods: Electrospun nanofibrous scaffolds based on polylactic acid/cellulose acetate (PLA/CA) or poly(caprolactone) (PCL) polymers were prepared and characterized. Different concentrations of green-synthesized silver nanoparticles, AgNPs (1-2% w/v) and hydroxyapatite nanoparticles, HANPs (10-20% w/v) were incorporated into the scaffolds to enhance the antibacterial and bone regeneration activity.
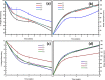
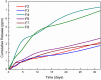
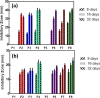
Results: In-vitro studies showed that addition of HANPs improved the cell viability by around 50% for both types of nanofibrous scaffolds. The tensile properties were also improved through addition of 10% HANPs but deteriorated upon increasing the concentration to 20%. AgNPs significantly improved the antibacterial activity with 40 mm inhibition zone after 32 days. Additionally, the nanofibrous scaffolds showed a desirable degradation profile with losing around 40-70% of its mass in 8 weeks.
Conclusions: The obtained results show that the developed nanofibrous membranes are promising scaffolds for both GTR and GBR applications.
Keywords: GBR; GTR; Nanofibers; Nanoparticles; Periodontal.
© 2020 The Authors. Published by Elsevier B.V. on behalf of Cairo University.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

