Static internal representation of dynamic situations reveals time compaction in human cognition
- PMID: 33364049
- PMCID: PMC7753960
- DOI: 10.1016/j.jare.2020.08.008
Static internal representation of dynamic situations reveals time compaction in human cognition
Abstract
Introduction: The human brain has evolved under the constraint of survival in complex dynamic situations. It makes fast and reliable decisions based on internal representations of the environment. Whereas neural mechanisms involved in the internal representation of space are becoming known, entire spatiotemporal cognition remains a challenge. Growing experimental evidence suggests that brain mechanisms devoted to spatial cognition may also participate in spatiotemporal information processing.
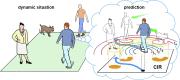
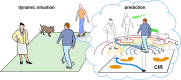
Objectives: The time compaction hypothesis postulates that the brain represents both static and dynamic situations as purely static maps. Such an internal reduction of the external complexity allows humans to process time-changing situations in real-time efficiently. According to time compaction, there may be a deep inner similarity between the representation of conventional static and dynamic visual stimuli. Here, we test the hypothesis and report the first experimental evidence of time compaction in humans.
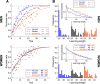
Methods: We engaged human subjects in a discrimination-learning task consisting in the classification of static and dynamic visual stimuli. When there was a hidden correspondence between static and dynamic stimuli due to time compaction, the learning performance was expected to be modulated. We studied such a modulation experimentally and by a computational model.
Results: The collected data validated the predicted learning modulation and confirmed that time compaction is a salient cognitive strategy adopted by the human brain to process time-changing situations. Mathematical modelling supported the finding. We also revealed that men are more prone to exploit time compaction in accordance with the context of the hypothesis as a cognitive basis for survival.
Conclusions: The static internal representation of dynamic situations is a human cognitive mechanism involved in decision-making and strategy planning to cope with time-changing environments. The finding opens a new venue to understand how humans efficiently interact with our dynamic world and thrive in nature.
Keywords: Decision making; Dynamic environments; Spatiotemporal cognition; Strategy planning.
© 2020 The Authors. Published by Elsevier B.V. on behalf of Cairo University.
Conflict of interest statement
None.
Figures






Similar articles
-
Spatial prediction of dynamic interactions in rats.PLoS One. 2025 Feb 25;20(2):e0319101. doi: 10.1371/journal.pone.0319101. eCollection 2025. PLoS One. 2025. PMID: 39999096 Free PMC article.
-
Semantic Knowledge Representation for Strategic Interactions in Dynamic Situations.Front Neurorobot. 2020 Feb 13;14:4. doi: 10.3389/fnbot.2020.00004. eCollection 2020. Front Neurorobot. 2020. PMID: 32116635 Free PMC article.
-
Minimal Neural Network Conditions for Encoding Future Interactions.Int J Neural Syst. 2025 Apr;35(4):2550016. doi: 10.1142/S0129065725500169. Epub 2025 Feb 28. Int J Neural Syst. 2025. PMID: 40019236
-
Human Representation Learning.Annu Rev Neurosci. 2021 Jul 8;44:253-273. doi: 10.1146/annurev-neuro-092920-120559. Epub 2021 Mar 17. Annu Rev Neurosci. 2021. PMID: 33730510 Free PMC article. Review.
-
Uncovering the 'state': Tracing the hidden state representations that structure learning and decision-making.Behav Processes. 2019 Oct;167:103891. doi: 10.1016/j.beproc.2019.103891. Epub 2019 Aug 2. Behav Processes. 2019. PMID: 31381985 Free PMC article. Review.
Cited by
-
Spatial prediction of dynamic interactions in rats.PLoS One. 2025 Feb 25;20(2):e0319101. doi: 10.1371/journal.pone.0319101. eCollection 2025. PLoS One. 2025. PMID: 39999096 Free PMC article.
-
Spatial Memory in a Spiking Neural Network with Robot Embodiment.Sensors (Basel). 2021 Apr 10;21(8):2678. doi: 10.3390/s21082678. Sensors (Basel). 2021. PMID: 33920246 Free PMC article.
-
Semantic Knowledge Representation for Strategic Interactions in Dynamic Situations.Front Neurorobot. 2020 Feb 13;14:4. doi: 10.3389/fnbot.2020.00004. eCollection 2020. Front Neurorobot. 2020. PMID: 32116635 Free PMC article.
-
Toward Reflective Spiking Neural Networks Exploiting Memristive Devices.Front Comput Neurosci. 2022 Jun 16;16:859874. doi: 10.3389/fncom.2022.859874. eCollection 2022. Front Comput Neurosci. 2022. PMID: 35782090 Free PMC article. Review.
References
-
- Eichenbaum H., Fortin N. Episodic memory and the hippocampus: It’s about time. Curr Dir Psichol Sci. 2003;12(2):53–57.
-
- Llinás R.R. MIT Press; 2001. I of the Vortex: From neurons to self.
-
- Rao S., Mayer A., Harrington D. The evolution of brain activation during temporal processing. Nat Neurosci. 2001;4:317–323. - PubMed
-
- Livesey A.C., Wall M.B., Smith A.T. Time perception: manipulation of task difficulty dissociates clock functions from other cognitive demands. Neuropsychologia. 2007;45:321–331. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

