Early label-free analysis of mitochondrial redox states by Raman spectroscopy predicts septic outcomes
- PMID: 33364057
- PMCID: PMC7753238
- DOI: 10.1016/j.jare.2020.06.027
Early label-free analysis of mitochondrial redox states by Raman spectroscopy predicts septic outcomes
Abstract
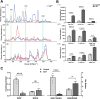
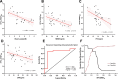
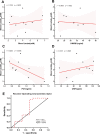
Background: Sepsis remains an unacceptably high mortality due to the lack of biomarkers for predicting septic outcomes in the early period. Mitochondrial redox states play a pivotal role in this condition and are disturbed early in the development of sepsis. Here, we hypothesized that visualizing mitochondrial redox states via resonance Raman spectroscopy (RRS) could identify septic outcomes at an early time point. Sepsis was induced by cecal ligation and puncture (CLP). We applied RRS analysis at baseline and 30 min, 1 h, 2 h, 4 h, and 6 h after CLP, and the mitochondrial redox states were identified. The levels of blood lactate as a predictor in sepsis were assessed. Our study is the first to reveal the possibility of in vivo detection of the mitochondrial redox state by using RRS in septic mice. The peak area for the Raman reduced mitochondrial fraction, the indicator of mitochondrial redox states, fluctuated significantly at 2 h after CLP. This fluctuation occurred earlier than the change in lactate level. Moreover, this fluctuation had higher prognostic accuracy for mortality than the lactate level during sepsis and could be a novel diagnostic marker for predicting septic outcomes according to the cutoff value of 1.059, which had a sensitivity of 80% and a specificity of 90%.
Objectives: To explore an effective indicator concerning mitochondrial redox states in the early stage of sepsis and to predict septic outcomes accurately in vivo using non-invasive and label-free Resonance Raman spectroscopy (RRS) analysis.
Methods: Mitochondria, primary skeletal muscle cells andex-vivo muscles harvested from gastrocnemius were detected mitochondrial redox states respectively by using RRS. Sepsis was induced by cecal ligation and puncture (CLP). We applied RRS analysis at baseline and 30 min, 1 h, 2 h, 4 h, and 6 h after CLP, and the mitochondrial redox states were identified. The levels of blood lactate as a predictor in sepsis were assessed. The predictive correlation of mitochondrial redox states on mortality, inflammation and organ dysfunction was further assessed.
Results: Mitochondrial redox states were clearly recognized in ex-vivo gastrocnemius muscles as well as purified mitochondria and primary skeletal muscle cells by using RRS. The peak area for the Raman reduced mitochondrial fraction, the indicator of mitochondrial redox states, fluctuated significantly at 2 h after CLP. This fluctuation occurred earlier than the change in lactate level. Moreover, this fluctuation had higher prognostic accuracy for mortality than the lactate level during sepsis and could be a novel diagnostic marker for predicting septic outcomes according to the cutoff value of 1.059, which had a sensitivity of 80% and a specificity of 90%.
Conclusions: This study demonstrated that monitoring mitochondrial redox states using RRS as early as 2 h could indicate outcomes in septic mice. These data may contribute to developing a non-invasive clinical device concerning mitochondrial redox states by using bedside-RRS.
Keywords: Inflammatory response; Label-free; Mitochondrial redox state; Multiple organ failure; Resonance Raman spectrum; Sepsis.
© 2020 The Authors. Published by Elsevier B.V. on behalf of Cairo University.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
An appraisal of studies using mouse models to assist the biomarker discovery for sepsis prognosis.Heliyon. 2023 Jun 28;9(7):e17770. doi: 10.1016/j.heliyon.2023.e17770. eCollection 2023 Jul. Heliyon. 2023. PMID: 37456011 Free PMC article. Review.
-
Label-free in vivo assessment of brain mitochondrial redox states during the development of diabetic cognitive impairment using Raman spectroscopy.Free Radic Biol Med. 2022 May 1;184:1-11. doi: 10.1016/j.freeradbiomed.2022.03.005. Epub 2022 Mar 24. Free Radic Biol Med. 2022. PMID: 35339608
-
Sepsis Disrupts Mitochondrial Function and Diaphragm Morphology.Front Physiol. 2021 Sep 7;12:704044. doi: 10.3389/fphys.2021.704044. eCollection 2021. Front Physiol. 2021. PMID: 34557108 Free PMC article.
-
Label-free analysis of the β-hydroxybutyricacid drug on mitochondrial redox states repairment in type 2 diabetic mice by resonance raman scattering.Biomed Pharmacother. 2024 Mar;172:116320. doi: 10.1016/j.biopha.2024.116320. Epub 2024 Feb 22. Biomed Pharmacother. 2024. PMID: 38387134
-
An Overview on Mitochondrial-Based Therapies in Sepsis-Related Myocardial Dysfunction: Mitochondrial Transplantation as a Promising Approach.Can J Infect Dis Med Microbiol. 2022 Jun 6;2022:3277274. doi: 10.1155/2022/3277274. eCollection 2022. Can J Infect Dis Med Microbiol. 2022. PMID: 35706715 Free PMC article. Review.
Cited by
-
An appraisal of studies using mouse models to assist the biomarker discovery for sepsis prognosis.Heliyon. 2023 Jun 28;9(7):e17770. doi: 10.1016/j.heliyon.2023.e17770. eCollection 2023 Jul. Heliyon. 2023. PMID: 37456011 Free PMC article. Review.
-
Arch watch: current approaches and opportunities for improvement.J Perinatol. 2024 Mar;44(3):325-332. doi: 10.1038/s41372-023-01854-7. Epub 2023 Dec 21. J Perinatol. 2024. PMID: 38129600 Review.
-
New Highly Sensitive and Specific Raman Probe for Live Cell Imaging of Mitochondrial Function.ACS Sens. 2024 Feb 23;9(2):995-1003. doi: 10.1021/acssensors.3c02576. Epub 2024 Feb 9. ACS Sens. 2024. PMID: 38334979 Free PMC article.
-
The future of acute and emergency care.Future Healthc J. 2021 Jul;8(2):e230-e236. doi: 10.7861/fhj.2021-0097. Future Healthc J. 2021. PMID: 34286190 Free PMC article.
-
DNA damage in preimplantation embryos and gametes: specification, clinical relevance and repair strategies.Hum Reprod Update. 2022 May 2;28(3):376-399. doi: 10.1093/humupd/dmab046. Hum Reprod Update. 2022. PMID: 35021196 Free PMC article. Review.
References
-
- Rhodes A., Evans L.E., Alhazzani W., Levy M.M., Antonelli M., Ferrer R. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45:486–552. - PubMed
-
- Fleischmann C., Scherag A., Adhikari N.K., Hartog C.S., Tsaganos T., Schlattmann P. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–272. - PubMed
-
- Fink M.P. Cytopathic hypoxia. Is oxygen use impaired in sepsis as a result of an acquired intrinsic derangement in cellular respiration? Crit Care Clin. 2002;18:165–175. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

