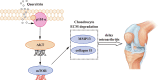
Quercitrin alleviates cartilage extracellular matrix degradation and delays ACLT rat osteoarthritis development: An in vivo and in vitro study
- PMID: 33364061
- PMCID: PMC7753236
- DOI: 10.1016/j.jare.2020.06.020
Quercitrin alleviates cartilage extracellular matrix degradation and delays ACLT rat osteoarthritis development: An in vivo and in vitro study
Abstract
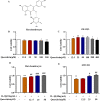
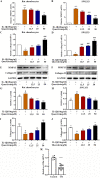
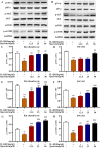
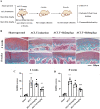
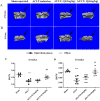
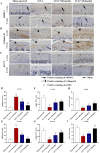
Introduction: Disruptions of extracellular matrix (ECM) degradation homeostasis play a significant role in the pathogenesis of osteoarthritis (OA). Matrix metalloproteinase 13 (MMP13) and collagen Ⅱ are important components of ECM. Earlier we found that quercitrin could significantly decrease MMP13 gene expression and increase collagen Ⅱ gene expression in IL-1β-induced rat chondrocytes and human chondrosarcoma (SW1353) cells. Objectives: The effects and mechanism of quercitrin on OA were explored. Methods: Molecular mechanisms of quercitrin on OA were studied in vitro in primary chondrocytes and SW1353 cells. An anterior cruciate ligament transection (ACLT) rat model of OA was used to investigate the effect of quercitrin in vivo. Micro-CT analysis and Safranin O-Fast Green Staining of knee joint samples were performed to observe the damage degree of tibial subchondral bone. Immunohistochemistry of knee joint samples were conducted to observe the protein level of MMP13, collagen Ⅱ and p110α in articular cartilage. Results: In vitro, quercitrin promoted cell proliferation and delayed ECM degradation by regulating MMP13 and collagen II gene and protein expressions. Moreover, quercitrin activated the Phosphatidylinositol 3-kinase p110α (p110α)/AKT/mTOR signaling pathway by targeting p110α. We also firstly showed that the gene expression level of p110α was remarkably decreased in cartilage of OA patients. The results showed that intra-articular injection of quercitrin increased bone volume/tissue volume of tibial subchondral bone and cartilage thickness and reduced the Osteoarthritis Research Society International scores in OA rats. Meanwhile, immunohistochemical results showed that quercitrin exerted anti-OA effect by delaying ECM degradation. Conclusion: These findings suggested that quercitrin may be a prospective disease-modifying OA drug for prevention and treatment of early stage OA.
Keywords: ACLT, anterior cruciate ligament transection; BV/TV, bone volume/tissue volume; DMOAD, disease-modifying OA drug; ECM, extracellular matrix; Extracellular matrix degradation; MMP13; MMP13, matrix metalloproteinase 13; NSAIDs, non-steroidal anti-inflammatory drugs; OA, osteoarthritis; OARSI, Osteoarthritis Research Society International; Osteoarthritis; PI3K, Phosphatidylinositol 3-kinase; Phosphatidylinositol 3-kinase p110α; Quercitrin; p110α, Phosphatidylinositol 3-kinase p110α.
© 2020 The Authors. Published by Elsevier B.V. on behalf of Cairo University.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Karsdal M.A., Michaelis M., Ladel C., Siebuhr A.S., Bihlet A.R., Andersen J.R. Disease-modifying treatments for osteoarthritis (Dmoads) of the knee and hip: lessons learned from failures and opportunities for the future. Osteoarthritis Cartilage. 2016;24(12):2013–2021. - PubMed
-
- Hunter D.J. Pharmacologic therapy for osteoarthritis–the era of disease modification. Nat Rev Rheumatol. 2011;7(1):13–22. - PubMed
-
- Glyn-Jones S., Palmer A.J.R., Agricola R., Price A.J., Vincent T.L., Weinans H. Osteoarthritis. The Lancet. 2015;386(9991):376–387. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

