Suprachoroidally Delivered DNA Nanoparticles Transfect Retina and Retinal Pigment Epithelium/Choroid in Rabbits
- PMID: 33364076
- PMCID: PMC7745627
- DOI: 10.1167/tvst.9.13.21
Suprachoroidally Delivered DNA Nanoparticles Transfect Retina and Retinal Pigment Epithelium/Choroid in Rabbits
Abstract
Purpose: This study evaluated ocular tolerability and transfectability of nonviral DNA nanoparticles (DNPs) after microneedle-based suprachoroidal (SC) administration, in comparison to subretinal (SR) administration.
Methods: The DNPs consisted of a single copy of plasmid DNA with a polyubiquitin C/luciferase transcriptional cassette compacted with 10 kDa PEG-substituted lysine 30-mer peptides (CK30PEG10k). New Zealand White rabbits (n = 4 per group) received a unilateral SC injection (0.1 mL via a microneedle technique) of ellipsoid-shaped DNPs, rod-shaped DNPs, or saline (negative control). A cohort of rabbits (n = 4) also received a single unilateral SR injection (0.05 mL via a transvitreal approach) of rod-shaped DNPs. At day 7, luciferase activity was measured in the retina and retinal pigment epithelium (RPE)-choroid via bioluminescence assay. A cohort of rabbits received a SC injection of analogous DNPs to assess spread of DNP injectate in the suprachoroidal space (SCS) via optical coherent tomography and histology.
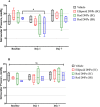
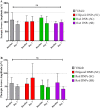
Results: Suprachoroidal injection of DNPs resulted in reversible opening of the SCS circumferentially and posteriorly and was generally well tolerated, with no significant ocular examination score changes, intraocular pressure abnormalities, or changes in electroretinography amplitudes on day 7 compared to the baseline. High luciferase activity was observed in the retina and RPE-choroid of eyes that received SC DNPs (rod and ellipsoid shape) and SR DNPs (rod shape) compared to controls. The mean luciferase activity in RPE-choroid and retina was comparable between SC and SR administrations. Transfection in the RPE-choroid was approximately 10-fold higher than in the retina after either SC or SR administration of DNPs.
Conclusions: Suprachoroidal and SR administration of DNPs resulted in comparable transfection of retina and RPE-choroid.
Translational relevance: Suprachoroidal delivery of DNPs offers the potential to precisely target chorioretinal tissues while avoiding surgical risks associated with SR injection, and it may offer an office-based nonsurgical gene therapy option for the treatment of retinal diseases.
Keywords: DNA nanoparticles; chorioretinal diseases; nonviral gene therapy; suprachoroidal delivery.
Copyright 2020 The Authors.
Conflict of interest statement
Disclosure: V.S. Kansara, Clearside Biomedical, Inc. (E, F); M. Cooper, Copernicus Therapeutics (E, F); O. Sesenoglu-Laird, Copernicus Therapeutics (E, F); L. Muya, Clearside Biomedical, Inc. (E, F); R. Moen, Copernicus Therapeutics (E, F); T.A. Ciulla, Clearside Biomedical, Inc. (E, F)
Figures





References
-
- US Food and Drug Administration. FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-novel-g.... Accessed June 3, 2020.
-
- Moore NA, Morral N, Ciulla TA, Bracha P. Gene therapy for inherited retinal and optic nerve degenerations. Expert Opin Biol Ther. 2018; 18(1): 37–49. - PubMed
-
- Seidman C, Kiss S. Gene therapy: the next frontier for treatment of acquired and inherited ocular disorders. Retina Today. 2015;69–71.
-
- Moore NA, Bracha P, Hussain RM, Morral N, Ciulla TA. Gene therapy for age-related macular degeneration. Expert Opin Biol Ther. 2017; 17(10): 1235–1244. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

