Microenvironmental Determinants of Breast Cancer Metastasis: Focus on the Crucial Interplay Between Estrogen and Insulin/Insulin-Like Growth Factor Signaling
- PMID: 33364239
- PMCID: PMC7753049
- DOI: 10.3389/fcell.2020.608412
Microenvironmental Determinants of Breast Cancer Metastasis: Focus on the Crucial Interplay Between Estrogen and Insulin/Insulin-Like Growth Factor Signaling
Abstract
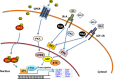
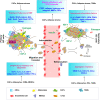
The development and progression of the great majority of breast cancers (BCs) are mainly dependent on the biological action elicited by estrogens through the classical estrogen receptor (ER), as well as the alternate receptor named G-protein-coupled estrogen receptor (GPER). In addition to estrogens, other hormones and growth factors, including the insulin and insulin-like growth factor system (IIGFs), play a role in BC. IIGFs cooperates with estrogen signaling to generate a multilevel cross-communication that ultimately facilitates the transition toward aggressive and life-threatening BC phenotypes. In this regard, the majority of BC deaths are correlated with the formation of metastatic lesions at distant sites. A thorough scrutiny of the biological and biochemical events orchestrating metastasis formation and dissemination has shown that virtually all cell types within the tumor microenvironment work closely with BC cells to seed cancerous units at distant sites. By establishing an intricate scheme of paracrine interactions that lead to the expression of genes involved in metastasis initiation, progression, and virulence, the cross-talk between BC cells and the surrounding microenvironmental components does dictate tumor fate and patients' prognosis. Following (i) a description of the main microenvironmental events prompting BC metastases and (ii) a concise overview of estrogen and the IIGFs signaling and their major regulatory functions in BC, here we provide a comprehensive analysis of the most recent findings on the role of these transduction pathways toward metastatic dissemination. In particular, we focused our attention on the main microenvironmental targets of the estrogen-IIGFs interplay, and we recapitulated relevant molecular nodes that orientate shared biological responses fostering the metastatic program. On the basis of available studies, we propose that a functional cross-talk between estrogens and IIGFs, by affecting the BC microenvironment, may contribute to the metastatic process and may be regarded as a novel target for combination therapies aimed at preventing the metastatic evolution.
Keywords: GPER; breast cancer; estrogen receptor; insulin/IGF signaling; metastasis; targeted therapies; tumor microenvionment.
Copyright © 2020 Vella, De Francesco, Lappano, Muoio, Manzella, Maggiolini and Belfiore.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

