NADPH performs mediated electron transfer in cyanobacterial-driven bio-photoelectrochemical cells
- PMID: 33364581
- PMCID: PMC7750406
- DOI: 10.1016/j.isci.2020.101892
NADPH performs mediated electron transfer in cyanobacterial-driven bio-photoelectrochemical cells
Abstract
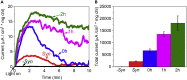
Previous studies have shown that live cyanobacteria can produce photocurrent in bio-photoelectrochemical cells (BPECs) that can be exploited for clean renewable energy production. Electron transfer from cyanobacteria to the electrochemical cell was proposed to be facilitated by small molecule(s) mediator(s) whose identity (or identities) remain unknown. Here, we elucidate the mechanism of electron transfer in the BPEC by identifying the major electron mediator as NADPH in three cyanobacterial species. We show that an increase in the concentration of NADPH secreted into the external cell medium (ECM) is obtained by both illumination and activation of the BPEC. Elimination of NADPH in the ECM abrogates the photocurrent while addition of exogenous NADP+ significantly increases and prolongs the photocurrent production. NADP+ is thus the first non-toxic, water soluble electron mediator that can functionally link photosynthetic cells to an energy conversion system and may serve to improve the performance of future BPECs.
Keywords: Bio-Electrochemistry; Biotechnology; Energy Engineering; Microbiology.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Batie C.J., Kamin H. Association of ferredoxin-NADP+ reductase with NADP(H) specificity and oxidation-reduction properties. J. Biol. Chem. 1986;261:11214–11223. - PubMed
-
- Bergel A., Féron D., Mollica A. Catalysis of oxygen reduction in PEM fuel cell by seawater biofilm. Electrochem. Commun. 2005;7:900–904.
-
- Blankenship R.E., Tiede D.M., Barber J., Brudvig G.W., Fleming G., Ghirardi M., Gunner M.R., Junge W., Kramer D.M., Melis A. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science. 2011;332:805–809. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

