Genetics of Anthracycline Cardiomyopathy in Cancer Survivors: JACC: CardioOncology State-of-the-Art Review
- PMID: 33364618
- PMCID: PMC7757557
- DOI: 10.1016/j.jaccao.2020.09.006
Genetics of Anthracycline Cardiomyopathy in Cancer Survivors: JACC: CardioOncology State-of-the-Art Review
Abstract
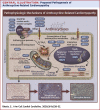
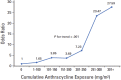
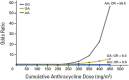
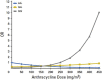
Anthracyclines are an integral part of chemotherapy regimens used to treat a variety of childhood-onset and adult-onset cancers. However, the development of cardiac dysfunction and heart failure often compromises the clinical utility of anthracyclines. The risk of cardiac dysfunction increases with anthracycline dose. This anthracycline-cardiac dysfunction association is modified by several demographic and clinical factors, such as age at anthracycline exposure (<4 years and ≥65 years); female sex; chest radiation; presence of cardiovascular risk factors (diabetes, hypertension); and concurrent use of cyclophosphamide, paclitaxel, and trastuzumab. However, the clinical variables alone yield modest predictive power in detecting cardiac dysfunction. Recently, attention has focused on the molecular basis of anthracycline-related cardiac dysfunction, providing an initial understanding of the mechanism of anthracycline-related cardiomyopathy. This review describes the current state of knowledge with respect to the pathogenesis of anthracycline-related cardiomyopathy and identifies the critical next steps to mitigate this problem.
Keywords: anthracycline chemotherapy; cardiomyopathy; genomics; heart failure; metabolomics; prediction.
Conflict of interest statement
Dr. Bhatia has received funding from the National Institutes of Health (R35CA220502), Leukemia Lymphoma Society (6563-19), and V Foundation (DT2019-010).
Figures


References
-
- Baech J., Hansen S.M., Lund P.E. Cumulative anthracycline exposure and risk of cardiotoxicity; a Danish nationwide cohort study of 2440 lymphoma patients treated with or without anthracyclines. Br J Haematol. 2018;183:717–726. - PubMed
-
- Chihara D., Westin J.R., Oki Y. Management strategies and outcomes for very elderly patients with diffuse large B-cell lymphoma. Cancer. 2016;122:3145–3151. - PubMed
-
- van Dalen E.C., van der Pal H.J., Kok W.E., Caron H.N., Kremer L.C. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer. 2006;42:3191–3198. - PubMed
-
- Krischer J.P., Epstein S., Cuthbertson D.D., Goorin A.M., Epstein M.L., Lipshultz S.E. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol. 1997;15:1544–1552. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

