The Acute Effects of 5 Fluorouracil on Skeletal Muscle Resident and Infiltrating Immune Cells in Mice
- PMID: 33364975
- PMCID: PMC7750461
- DOI: 10.3389/fphys.2020.593468
The Acute Effects of 5 Fluorouracil on Skeletal Muscle Resident and Infiltrating Immune Cells in Mice
Abstract
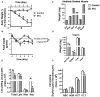
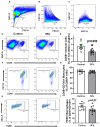
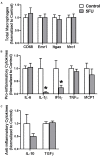
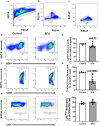
5 fluorouracil (5FU) has been a first-choice chemotherapy drug for several cancer types (e.g., colon, breast, head, and neck); however, its efficacy is diminished by patient acquired resistance and pervasive side effects. Leukopenia is a hallmark of 5FU; however, the impact of 5FU-induced leukopenia on healthy tissue is only becoming unearthed. Recently, skeletal muscle has been shown to be impacted by 5FU in clinical and preclinical settings and weakness and fatigue remain among the most consistent complaints in cancer patients undergoing chemotherapy. Monocytes, or more specifically macrophages, are the predominate immune cell in skeletal muscle which regulate turnover and homeostasis through removal of damaged or old materials as well as coordinate skeletal muscle repair and remodeling. Whether 5FU-induced leukopenia extends beyond circulation to impact resident and infiltrating skeletal muscle immune cells has not been examined. The purpose of the study was to examine the acute effects of 5FU on resident and infiltrating skeletal muscle monocytes and inflammatory mediators. Male C57BL/6 mice were given a physiologically translatable dose (35 mg/kg) of 5FU, or PBS, i.p. once daily for 5 days to recapitulate 1 dosing cycle. Our results demonstrate that 5FU reduced circulating leukocytes, erythrocytes, and thrombocytes while inducing significant body weight loss (>5%). Flow cytometry analysis of the skeletal muscle indicated a reduction in total CD45+ immune cells with a corresponding decrease in total CD45+CD11b+ monocytes. There was a strong relationship between circulating leukocytes and skeletal muscle CD45+ immune cells. Skeletal muscle Ly6cHigh activated monocytes and M1-like macrophages were reduced with 5FU treatment while total M2-like CD206+CD11c- macrophages were unchanged. Interestingly, 5FU reduced bone marrow CD45+ immune cells and CD45+CD11b+ monocytes. Our results demonstrate that 5FU induced body weight loss and decreased skeletal muscle CD45+ immune cells in association with a reduction in infiltrating Ly6cHigh monocytes. Interestingly, the loss of skeletal muscle immune cells occurred with bone marrow cell cycle arrest. Together our results highlight that skeletal muscle is sensitive to 5FU's off-target effects which disrupts both circulating and skeletal muscle immune cells.
Keywords: bone marrow; chemotherapy; macrophages; monocytes; skeletal muscle.
Copyright © 2020 VanderVeen, Sougiannis, Velazquez, Carson, Fan and Murphy.
Conflict of interest statement
BV, DF, and EM were employed by the company AcePre LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

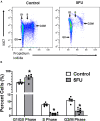
References
-
- Abraham J. E., Hiller L., Dorling L., Vallier A. L., Dunn J., Bowden S., et al. (2015). A nested cohort study of 6,248 early breast cancer patients treated in neoadjuvant and adjuvant chemotherapy trials investigating the prognostic value of chemotherapy-related toxicities. BMC Med. 13:306. 10.1186/s12916-015-0547-5 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

