Direct and Indirect Effects of Sex Steroids on Gonadotrope Cell Plasticity in the Teleost Fish Pituitary
- PMID: 33365013
- PMCID: PMC7750530
- DOI: 10.3389/fendo.2020.605068
Direct and Indirect Effects of Sex Steroids on Gonadotrope Cell Plasticity in the Teleost Fish Pituitary
Abstract
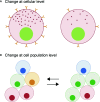
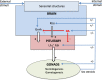
The pituitary gland controls many important physiological processes in vertebrates, including growth, homeostasis, and reproduction. As in mammals, the teleost pituitary exhibits a high degree of plasticity. This plasticity permits changes in hormone production and secretion necessary to meet the fluctuating demands over the life of an animal. Pituitary plasticity is achieved at both cellular and population levels. At the cellular level, hormone synthesis and release can be regulated via changes in cell composition to modulate both sensitivity and response to different signals. At the cell population level, the number of cells producing a given hormone can change due to proliferation, differentiation of progenitor cells, or transdifferentiation of specific cell types. Gonadotropes, which play an important role in the control of reproduction, have been intensively investigated during the last decades and found to display plasticity. To ensure appropriate endocrine function, gonadotropes rely on external and internal signals integrated at the brain level or by the gonadotropes themselves. One important group of internal signals is the sex steroids, produced mainly by the gonadal steroidogenic cells. Sex steroids have been shown to exert complex effects on the teleost pituitary, with differential effects depending on the species investigated, physiological status or sex of the animal, and dose or method of administration. This review summarizes current knowledge of the effects of sex steroids (androgens and estrogens) on gonadotrope cell plasticity in teleost anterior pituitary, discriminating direct from indirect effects.
Keywords: adenohypophysis; androgen; brain; estrogen; gonads; pituitary; plasticity; steroids.
Copyright © 2020 Fontaine, Royan, von Krogh, Weltzien and Baker.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Gonadotrope plasticity at cellular, population and structural levels: A comparison between fishes and mammals.Gen Comp Endocrinol. 2020 Feb 1;287:113344. doi: 10.1016/j.ygcen.2019.113344. Epub 2019 Nov 30. Gen Comp Endocrinol. 2020. PMID: 31794734 Review.
-
Sexually Dimorphic Regulation of Gonadotrope Cell Hyperplasia in Medaka Pituitary via Mitosis and Transdifferentiation.Endocrinology. 2023 Feb 11;164(4):bqad030. doi: 10.1210/endocr/bqad030. Endocrinology. 2023. PMID: 36791137 Free PMC article.
-
Direct control of the gonadotroph in a teleost, Poecilia latipinna: gonadal steroids.Gen Comp Endocrinol. 1986 Mar;61(3):402-16. doi: 10.1016/0016-6480(86)90226-1. Gen Comp Endocrinol. 1986. PMID: 3956992
-
Plasticity of Anterior Pituitary Gonadotrope Cells Facilitates the Pre-Ovulatory LH Surge.Front Endocrinol (Lausanne). 2021 Feb 4;11:616053. doi: 10.3389/fendo.2020.616053. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33613451 Free PMC article. Review.
-
[Specific role of sex steroids on the pituitary level. Sex steroids and anterior pituitary secretion].Bull Schweiz Akad Med Wiss. 1978 Mar;34(1-3):171-90. Bull Schweiz Akad Med Wiss. 1978. PMID: 208692 French.
Cited by
-
Identification of the FSH-RH as the other gonadotropin-releasing hormone.Nat Commun. 2024 Jun 27;15(1):5342. doi: 10.1038/s41467-024-49564-8. Nat Commun. 2024. PMID: 38937445 Free PMC article.
-
Impact of germ cell ablation on the activation of the brain-pituitary-gonadal axis in precocious Atlantic salmon (Salmo salar L.) males.Mol Reprod Dev. 2022 Oct;89(10):471-484. doi: 10.1002/mrd.23635. Epub 2022 Jul 13. Mol Reprod Dev. 2022. PMID: 35830347 Free PMC article.
-
Day length regulates gonadotrope proliferation and reproduction via an intra-pituitary pathway in the model vertebrate Oryzias latipes.Commun Biol. 2024 Mar 30;7(1):388. doi: 10.1038/s42003-024-06059-y. Commun Biol. 2024. PMID: 38553567 Free PMC article.
-
Gene Editing of the Follicle-Stimulating Hormone Gene to Sterilize Channel Catfish, Ictalurus punctatus, Using a Modified Transcription Activator-like Effector Nuclease Technology with Electroporation.Biology (Basel). 2023 Mar 1;12(3):392. doi: 10.3390/biology12030392. Biology (Basel). 2023. PMID: 36979084 Free PMC article.
-
Pituitary Gonadotropin Gene Expression During Induced Onset of Postsmolt Maturation in Male Atlantic Salmon: In Vivo and Tissue Culture Studies.Front Endocrinol (Lausanne). 2022 Mar 15;13:826920. doi: 10.3389/fendo.2022.826920. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35370944 Free PMC article.
References
-
- Nelson JS. Fishes of the world. New Jersey, USA: John Wiley and Sons; (2006).
-
- Meyers JR. Zebrafish: Development of a vertebrate model organism. Curr Protoc Essential Lab Techniq (2018) 16(1):e19. 10.1002/cpet.19 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

