Icariin enhances cell survival in lipopolysaccharide-induced synoviocytes by suppressing ferroptosis via the Xc-/GPX4 axis
- PMID: 33365072
- PMCID: PMC7716635
- DOI: 10.3892/etm.2020.9504
Icariin enhances cell survival in lipopolysaccharide-induced synoviocytes by suppressing ferroptosis via the Xc-/GPX4 axis
Abstract
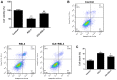
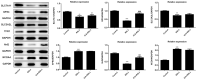
The mechanism of action of synovitis, as the vital pathological process of rheumatoid arthritis and osteoarthritis, remains to be elucidated. The effects and the mechanism of icariin (ICA), which is a promising therapeutic agent in synovitis, was investigated in the present study. In addition, ferroptosis, a vital cell process involved in several diseases, was also studied in synovitis for the first time. Lipopolysaccharide (LPS)-induced synoviocytes served as a synovitis cell model. The cells were divided into control, LPS and experimental groups and were treated with different concentrations of ICA. Cell viability was determined by Cell Counting Kit-8 assay and cell death was determined by flow cytometry. The expression levels of proteins (GPX4, SLC7A11, SLC3A2L, TRF, Nrf2 and NCOA4) were measured by western blotting. Quantification of malondialdehyde (MDA), iron and glutathione peroxidase 4 (GPX4) activity levels were performed via using corresponding assay kits. Cell death was increased, and cell viability was decreased in LPS-induced synoviocytes. Furthermore, MDA levels and iron content were elevated and GPX levels was reduced in LPS-induced synoviocytes. Transferrin receptor protein 1 and nuclear receptor coactivator 4 were upregulated and proteins of the Xc-/GPX4 axis, as well as nuclear factor erythroid 2-related factor 2, were decreased by LPS treatment. All aforementioned LPS affects were alleviated by ICA via a concentration-dependent manner. ICA counteracted the effects of RSL3, a ferroptosis activator, on cell viability, lipid peroxidation, iron content and relative protein expression of ferroptosis in synoviocytes. ICA protects the cells from death in synoviocytes induced by LPS, via the inhibition of ferroptosis by activating the Xc-/GPX4 axis, which can be exploited as a new therapeutic strategy for synovitis.
Keywords: Xc-/glutathione peroxidase 4 axis; ferroptosis; icariin; synoviocytes; synovitis.
Copyright © 2020, Spandidos Publications.
Figures



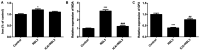
References
-
- Safiri S, Kolahi AA, Hoy D, Smith E, Bettampadi D, Mansournia MA, Almasi-Hashiani A, Ashrafi-Asgarabad A, Moradi-Lakeh M, Qorbani M, et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: A systematic analysis of the global burden of disease study 2017. Ann Rheum Dis. 2019;78:1463–1471. doi: 10.1136/annrheumdis-2019-215920. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
