Human exhaled air can efficiently support in vitro maturation of porcine oocytes and subsequent early embryonic development
- PMID: 33365092
- PMCID: PMC7746221
- DOI: 10.21451/1984-3143-AR2017-0027
Human exhaled air can efficiently support in vitro maturation of porcine oocytes and subsequent early embryonic development
Abstract
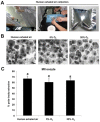
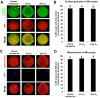
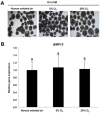
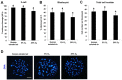
Air phase is an indispensable environmental factor affecting oocyte maturation and early embryo development. Human exhaled air was previously proved to be a reliable and inexpensive atmosphere that sustains normal early development of mouse and bovine embryos. However, whether human exhaled air can support in vitro maturation (IVM) of porcine oocytes is not yet known. To evaluate the feasibility of maturing oocytes in human exhaled air, we examined oocyte morphology, BMP15 expression, nuclear and cytoplasmic maturation. We found that cumulus expansion status, expression levels of BMP15 important for cumulus expansion and the rate of first polar body emission were similar among human exhaled air, 5% O2 or 20% O2 in air after IVM of 44 h. Furthermore, the percentage of metaphase II (MII) oocytes showing normal cortical and sub-membranous localization of cortical granules and diffused mitochondrial distribution patterns is comparable among groups. The cleavage, blastocyst rate and total cell number were not apparently different for parthenogenetic activated and somatic cloned embryos derived from MII oocytes matured in three air phases, suggesting oocytes matured in human exhaled air obtain normal developmental competence. Taken together, human exhaled air can efficiently support in vitro maturation of porcine oocytes and subsequent early embryonic development.
Keywords: early embryo; human exhaled air; oocyte maturation; pig; somatic cell nuclear transfer.
Conflict of interest statement
Conflicts of Interest The authors declare no competing or financial interests in this research.
Figures

Similar articles
-
A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence.Mol Hum Reprod. 2017 Sep 1;23(9):594-606. doi: 10.1093/molehr/gax032. Mol Hum Reprod. 2017. PMID: 28586460
-
The promotory effect of growth hormone on the developmental competence of in vitro matured bovine oocytes is due to improved cytoplasmic maturation.Mol Reprod Dev. 1998 Apr;49(4):444-53. doi: 10.1002/(SICI)1098-2795(199804)49:4<444::AID-MRD12>3.0.CO;2-U. Mol Reprod Dev. 1998. PMID: 9508096
-
The new system of shorter porcine oocyte in vitro maturation (18 hours) using ≥8 mm follicles derived from cumulus-oocyte complexes.Theriogenology. 2014 Jan 15;81(2):291-301. doi: 10.1016/j.theriogenology.2013.09.028. Epub 2013 Oct 17. Theriogenology. 2014. PMID: 24220361
-
Improved cryotolerance and developmental potential of in vitro and in vivo matured mouse oocytes by supplementing with a glutathione donor prior to vitrification.Mol Hum Reprod. 2016 Dec;22(12):867-881. doi: 10.1093/molehr/gaw059. Epub 2016 Sep 7. Mol Hum Reprod. 2016. PMID: 27604460
-
Despite the donor's age, human adipose-derived stem cells enhance the maturation and development rates of porcine oocytes in a co-culture system.Theriogenology. 2018 Jul 15;115:57-64. doi: 10.1016/j.theriogenology.2017.12.024. Epub 2017 Dec 12. Theriogenology. 2018. PMID: 29709724 Review.
Cited by
-
The combination of rolipram and cilostamide improved the developmental competence of cloned porcine embryos.Sci Rep. 2023 Apr 7;13(1):5733. doi: 10.1038/s41598-023-32677-3. Sci Rep. 2023. PMID: 37029228 Free PMC article.
References
-
- Adam AA , Takahashi Y , Katagiri S , Nagano M. Effects of oxygen tension in the gas atmosphere during in vitro maturation, in vitro fertilization and in vitro culture on the efficiency of in vitro production of mouse embryos . Jpn J Vet Res. 2004;52:77–84. - PubMed
-
- Bavister BD. Culture of preimplantation embryos: facts and artifacts . Hum Reprod Update. 1995;1:91–148. - PubMed
-
- Bavister BD , Poole KA. Duration and temperature of culture medium equilibration affect frequency of blastocyst development . Reprod Biomed Online. 2005;10:124–129. - PubMed
-
- Brevini TA , Vassena R , Francisci C , Gandolfi F. Role of adenosine triphosphate, active mitochondria, and microtubules in the acquisition of developmental competence of parthenogenetically activated pig oocytes . Biol Reprod. 2005;72:1218–1223. - PubMed
-
- Brevini TA , Cillo F , Antonini S , Gandolfi F. Cytoplasmic remodelling and the acquisition of developmental competence in pig oocytes . Anim Reproduction Sci. 2007;98:23–38. - PubMed
LinkOut - more resources
Full Text Sources
