Study on the Occurrence of Genetic Exchange Among Parasites of the Leishmania mexicana Complex
- PMID: 33365278
- PMCID: PMC7750183
- DOI: 10.3389/fcimb.2020.607253
Study on the Occurrence of Genetic Exchange Among Parasites of the Leishmania mexicana Complex
Abstract
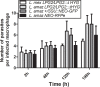
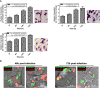
In Leishmania, genetic exchange has been experimentally demonstrated to occur in the sand fly vector and in promastigote axenic cultures through a meiotic-like process. No evidence of genetic exchange in mammalian hosts have been reported so far, possibly due to the fact that the Leishmania species used in previous studies replicate within individual parasitophorous vacuoles. In the present work, we explored the possibility that residing in communal vacuoles may provide conditions favorable for genetic exchange for L. mexicana and L. amazonensis. Using promastigote lines of both species harboring integrated or episomal drug-resistance markers, we assessed whether genetic exchange can occur in axenic cultures, in infected macrophages as well as in infected mice. We obtained evidence of genetic exchange for L. amazonensis in both axenic promastigote cultures and infected macrophages. However, the resulting products of those putative genetic events were unstable as they did not sustain growth in subsequent sub-cultures, precluding further characterization.
Keywords: Leishmania; drug resistance; genetic exchange; host-pathogen relationship; intracellular pathogen; macrophage.
Copyright © 2020 Telittchenko and Descoteaux.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Banuls A. L., Guerrini F., Le Pont F., Barrera C., Espinel I., Guderian R., et al. (1997). Evidence for hybridization by multilocus enzyme electrophoresis and random amplified polymorphic DNA between Leishmania braziliensis and Leishmania panamensis/guyanensis in Ecuador. J. Eukaryot. Microbiol. 44 (5), 408–411. 10.1111/j.1550-7408.1997.tb05716.x - DOI - PubMed
-
- Banuls A. L., Jonquieres R., Guerrini F., Le Pont F., Barrera C., Espinel I., et al. (1999). Genetic analysis of leishmania parasites in Ecuador: are Leishmania (Viannia) panamensis and Leishmania (V.) Guyanensis distinct taxa? Am. J. Trop. Med. Hyg. 61 (5), 838–845. 10.4269/ajtmh.1999.61.838 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

