Acetylcholine Regulates Pulmonary Pathology During Viral Infection and Recovery
- PMID: 33365281
- PMCID: PMC7751717
- DOI: 10.2147/ITT.S279228
Acetylcholine Regulates Pulmonary Pathology During Viral Infection and Recovery
Abstract
Introduction: This study was designed to explore the role of acetylcholine (ACh) in pulmonary viral infection and recovery. Inflammatory control is critical to recovery from respiratory viral infection. ACh secreted from non-neuronal sources, including lymphocytes, plays an important, albeit underappreciated, role in regulating immune-mediated inflammation.
Methods: ACh and lymphocyte cholinergic status in the lungs were measured over the course of influenza infection and recovery. The role of ACh was examined by inhibiting ACh synthesis in vivo. Pulmonary inflammation was monitored by Iba1 immunofluorescence, using a novel automated algorithm. Tissue repair was monitored histologically.
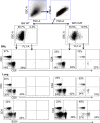
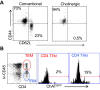
Results: Pulmonary ACh remained constant through the early stage of infection and increased during the peak of the acquired immune response. As the concentration of ACh increased, cholinergic lymphocytes appeared in the BAL and lungs. Cholinergic capacity was found primarily in CD4 T cells, but also in B cells and CD8 T cells. The cholinergic CD4+ T cells bound to influenza-specific tetramers and were retained in the resident memory regions of the lung up to 2 months after infection. Histologically, cholinergic lymphocytes were found in direct physical contact with activated macrophages throughout the lung. Inflammation was monitored by ionized calcium-binding adapter molecule 1 (Iba1) immunofluorescence, using a novel automated algorithm. When ACh production was inhibited, mice exhibited increased tissue inflammation and delayed recovery. Histologic examination revealed abnormal tissue repair when ACh was limited.
Conclusion: These findings point to a previously unrecognized role for ACh in the transition from active immunity to recovery and pulmonary repair following respiratory viral infection.
Keywords: Aif-1; CD4 resident memory; ChAT; Iba1; MATLAB; acetylcholine; acetylcholinesterase; automated algorithm; cholinergic anti-inflammatory pathway; cholinergic lymphocytes; inflammaging; inflammation; influenza; pulmonary repair.
© 2020 Horkowitz et al.
Conflict of interest statement
This study was supported by NIH AI119929 (JP), San Diego State University Bridge Funding (JP), and San Diego State University Initiative for Maximizing Student Development Award GM058906 (CA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors report no conflicts of interest in this work.
Figures






References
-
- Fujii T, Tsuchiya T, Yamada S, et al. Localization and synthesis of acetylcholine in human leukemic T cell lines. J Neurosci Res. 1996;44(1):66–72. - PubMed
-
- Kawashima K, Fujii T, Moriwaki Y, Misawa H, Horiguchi K. Non-neuronal cholinergic system in regulation of immune function with a focus on alpha7 nAChRs. Int Immunopharmacol. 2015;29(1):127–134. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

