Cohesin Components Stag1 and Stag2 Differentially Influence Haematopoietic Mesoderm Development in Zebrafish Embryos
- PMID: 33365313
- PMCID: PMC7750468
- DOI: 10.3389/fcell.2020.617545
Cohesin Components Stag1 and Stag2 Differentially Influence Haematopoietic Mesoderm Development in Zebrafish Embryos
Abstract
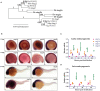
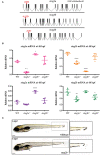
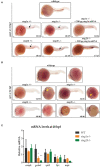
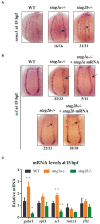
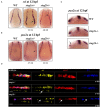
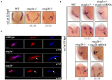
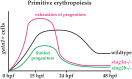
Cohesin is a multiprotein complex made up of core subunits Smc1, Smc3, and Rad21, and either Stag1 or Stag2. Normal haematopoietic development relies on crucial functions of cohesin in cell division and regulation of gene expression via three-dimensional chromatin organization. Cohesin subunit STAG2 is frequently mutated in myeloid malignancies, but the individual contributions of Stag variants to haematopoiesis or malignancy are not fully understood. Zebrafish have four Stag paralogues (Stag1a, Stag1b, Stag2a, and Stag2b), allowing detailed genetic dissection of the contribution of Stag1-cohesin and Stag2-cohesin to development. Here we characterize for the first time the expression patterns and functions of zebrafish stag genes during embryogenesis. Using loss-of-function CRISPR-Cas9 zebrafish mutants, we show that stag1a and stag2b contribute to primitive embryonic haematopoiesis. Both stag1a and stag2b mutants present with erythropenia by 24 h post-fertilization. Homozygous loss of either paralogue alters the number of haematopoietic/vascular progenitors in the lateral plate mesoderm. The lateral plate mesoderm zone of scl-positive cells is expanded in stag1a mutants with concomitant loss of kidney progenitors, and the number of spi1-positive cells are increased, consistent with skewing toward primitive myelopoiesis. In contrast, stag2b mutants have reduced haematopoietic/vascular mesoderm and downregulation of primitive erythropoiesis. Our results suggest that Stag1 and Stag2 proteins cooperate to balance the production of primitive haematopoietic/vascular progenitors from mesoderm.
Keywords: STAG1; STAG2; cohesin; development; haematopoiesis; mesoderm; zebrafish.
Copyright © 2020 Ketharnathan, Labudina and Horsfield.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

