No Home without Hormones: How Plant Hormones Control Legume Nodule Organogenesis
- PMID: 33367261
- PMCID: PMC7747975
- DOI: 10.1016/j.xplc.2020.100104
No Home without Hormones: How Plant Hormones Control Legume Nodule Organogenesis
Abstract
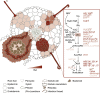
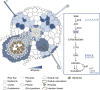
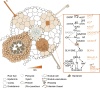
The establishment of symbiotic nitrogen fixation requires the coordination of both nodule development and infection events. Despite the evolution of a variety of anatomical structures, nodule organs serve a common purpose in establishing a localized area that facilitates efficient nitrogen fixation. As in all plant developmental processes, the establishment of a new nodule organ is regulated by plant hormones. During nodule initiation, regulation of plant hormone signaling is one of the major targets of symbiotic signaling. We review the role of major developmental hormones in the initiation of the nodule organ and argue that the manipulation of plant hormones is a key requirement for engineering nitrogen fixation in non-legumes as the basis for improved food security and sustainability.
Keywords: hormones; legume; nitrogen fixation; nodule; symbiosis.
© 2020 The Author(s).
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

