Milk exosomes with enhanced mucus penetrability for oral delivery of siRNA
- PMID: 33367332
- PMCID: PMC8205963
- DOI: 10.1039/d0bm01497d
Milk exosomes with enhanced mucus penetrability for oral delivery of siRNA
Abstract
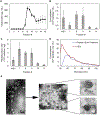
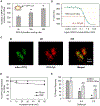
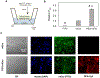
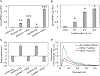
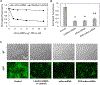
Bovine milk-derived exosomes have recently emerged as a promising nano-vehicle for the encapsulation and delivery of macromolecular biotherapeutics. Here we engineer high purity bovine milk exosomes (mExo) with modular surface tunability for oral delivery of small interfering RNA (siRNA). We utilize a low-cost enrichment method combining casein chelation with differential ultracentrifugation followed by size exclusion chromatography, yielding mExo of high concentration and purity. Using in vitro models, we demonstrate that negatively charged hydrophobic mExos can penetrate multiple biological barriers to oral drug delivery. A hydrophilic polyethylene glycol (PEG) coating was introduced on the mExo surface via passive, stable hydrophobic insertion of a conjugated lipid tail, which significantly reduced mExo degradation in acidic gastric environment and enhanced their permeability through mucin by over 3× compared to unmodified mExo. Both mExo and PEG-mExo exhibited high uptake by intestinal epithelial cells and mediated functional intracellular delivery of siRNA, thereby suppressing the expression of the target green fluorescence protein (GFP) gene by up to 70%. We also show that cationic chemical transfection is significantly more efficient in loading siRNA into mExo than electroporation. The simplicity of isolating high purity mExo in high concentrations and equipping them with tunable surface properties, demonstrated here, paves way for the development of mExo as an effective, scalable platform technology for oral drug delivery of siRNA.
Conflict of interest statement
Conflicts of Interest
There are no conflicts to declare.
Figures

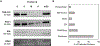

References
-
- Odom EBP, K.B.; Odom DC, Clinical Research, 2016, 8.
-
- Shepard J, Ward W, Milstone A, Carlson T, Frederick J, Hadhazy E and Perl T, JAMA Surg, 2013, 148, 907–914. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

