The cyanobacterial lectin, microvirin-N, enhances the specificity and sensitivity of lipoarabinomannan-based TB diagnostic tests
- PMID: 33367346
- PMCID: PMC8374243
- DOI: 10.1039/d0an01725f
The cyanobacterial lectin, microvirin-N, enhances the specificity and sensitivity of lipoarabinomannan-based TB diagnostic tests
Abstract
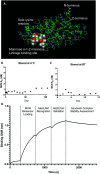
Tuberculosis (TB) is one of the top ten causes of death globally, despite being treatable. The eradication of TB disease requires, amongst others, diagnostic tests with high specificity and sensitivity that will work at the point of care (POC) in low-resource settings. The TB surface glycolipid antigen, mannose-capped lipoarabinomannan (ManLAM) currently serves as the only POC molecular diagnostic biomarker suitable for use in low cost immunoassays. Here, we demonstrate the high affinity and exceptional specificity of microvirin-N (MVN), a 14.3 kDa cyanobacterial lectin, toward H37Rv TB ManLAM and utilize it to develop a novel on-bead ELISA. MVN binds to ManLAM with sub-picomolar binding affinity, but does not bind to other variants of LAM expressed by non-pathogenic mycobacteria - a level of binding specificity and affinity that current commercially available anti-LAM antibodies cannot achieve. An on-bead ELISA was subsequently developed using MVN-functionalized magnetic beads which allows for the specific capture of ManLAM from human urine with a limit of detection (LOD) of 1.14 ng mL-1 and no cross-reactivity when tested with PILAM, a variant of LAM found on non-pathogenic mycobacteria.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


References
-
- World Health Organization, Global Tuberculosis Report 2018, World Health Organization, S.l., 2018
-
- Pai M. Nicol M. P. Boehme C. C. Microbiol. Spectrum. 2016;4:5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

