Vestibular agnosia in traumatic brain injury and its link to imbalance
- PMID: 33367536
- PMCID: PMC7880674
- DOI: 10.1093/brain/awaa386
Vestibular agnosia in traumatic brain injury and its link to imbalance
Abstract
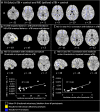
Vestibular dysfunction, causing dizziness and imbalance, is a common yet poorly understood feature in patients with TBI. Damage to the inner ear, nerve, brainstem, cerebellum and cerebral hemispheres may all affect vestibular functioning, hence, a multi-level assessment-from reflex to perception-is required. In a previous report, postural instability was the commonest neurological feature in ambulating acute patients with TBI. During ward assessment, we also frequently observe a loss of vertigo sensation in patients with acute TBI, common inner ear conditions and a related vigorous vestibular-ocular reflex nystagmus, suggesting a 'vestibular agnosia'. Patients with vestibular agnosia were also more unbalanced; however, the link between vestibular agnosia and imbalance was confounded by the presence of inner ear conditions. We investigated the brain mechanisms of imbalance in acute TBI, its link with vestibular agnosia, and potential clinical impact, by prospective laboratory assessment of vestibular function, from reflex to perception, in patients with preserved peripheral vestibular function. Assessment included: vestibular reflex function, vestibular perception by participants' report of their passive yaw rotations in the dark, objective balance via posturography, subjective symptoms via questionnaires, and structural neuroimaging. We prospectively screened 918 acute admissions, assessed 146 and recruited 37. Compared to 37 matched controls, patients showed elevated vestibular-perceptual thresholds (patients 12.92°/s versus 3.87°/s) but normal vestibular-ocular reflex thresholds (patients 2.52°/s versus 1.78°/s). Patients with elevated vestibular-perceptual thresholds [3 standard deviations (SD) above controls' average], were designated as having vestibular agnosia, and displayed worse posturography than non-vestibular-agnosia patients, despite no difference in vestibular symptom scores. Only in patients with impaired postural control (3 SD above controls' mean), whole brain diffusion tensor voxel-wise analysis showed elevated mean diffusivity (and trend lower fractional anisotropy) in the inferior longitudinal fasciculus in the right temporal lobe that correlated with vestibular agnosia severity. Thus, impaired balance and vestibular agnosia are co-localized to the inferior longitudinal fasciculus in the right temporal lobe. Finally, a clinical audit showed a sevenfold reduction in clinician recognition of a common peripheral vestibular condition (benign paroxysmal positional vertigo) in acute patients with clinically apparent vestibular agnosia. That vestibular agnosia patients show worse balance, but without increased dizziness symptoms, explains why clinicians may miss treatable vestibular diagnoses in these patients. In conclusion, vestibular agnosia mediates imbalance in traumatic brain injury both directly via white matter tract damage in the right temporal lobe, and indirectly via reduced clinical recognition of common, treatable vestibular diagnoses.
Keywords: self-motion perception; traumatic brain injury; vertigo; vestibular agnosia; vestibular cognition.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
-
- Agosta F, Galantucci S, Svetel M, Lukić MJ, Copetti M, Davidovic K, et al. Clinical, cognitive, and behavioural correlates of white matter damage in progressive supranuclear palsy. J Neurol 2014; 261: 913–24. - PubMed
-
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 2003; 50: 1077–88. - PubMed
-
- Brandt T, Strupp M, Benson J.. You are better off running than walking with acute vestibulopathy. Lancet 1999; 354: 746. - PubMed
-
- Chamelian L, Feinstein A.. Outcome after mild to moderate traumatic brain injury: the role of dizziness. Arch Phys Med Rehabil 2004; 85: 1662–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

