Field Performance and Public Health Response Using the BinaxNOWTM Rapid Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antigen Detection Assay During Community-Based Testing
- PMID: 33367619
- PMCID: PMC7799223
- DOI: 10.1093/cid/ciaa1890
Field Performance and Public Health Response Using the BinaxNOWTM Rapid Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antigen Detection Assay During Community-Based Testing
Abstract
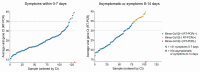
Among 3302 persons tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by BinaxNOWTM and reverse transcription polymerase chain reaction (RT-PCR) in a community setting, rapid assay sensitivity was 100%/98.5%/89% using RT-PCR cycle thresholds of 30, 35, and no threshold. The specificity was 99.9%. Performance was high across ages and those with and without symptoms. Rapid resulting permitted immediate public health action.
Keywords: asymptomatic SARS-CoV-2 infection; community-based SARS-CoV-2 testing.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- Unidos en Salud. Binax training resources. Available at: https://unitedinhealth.org/binax-training. Accessed 17 December 2020.
-
- BinaxNOWTM COVID-19 Ag card and NAVICATM App set-up and training. Available at: https://www.globalpointofcare.abbott/en/support/product-installation-tra.... Accessed 17 December 2020.

