Effect of Antigenic Drift on Influenza Vaccine Effectiveness in the United States-2019-2020
- PMID: 33367650
- PMCID: PMC8664438
- DOI: 10.1093/cid/ciaa1884
Effect of Antigenic Drift on Influenza Vaccine Effectiveness in the United States-2019-2020
Abstract
Background: At the start of the 2019-2020 influenza season, concern arose that circulating B/Victoria viruses of the globally emerging clade V1A.3 were antigenically drifted from the strain included in the vaccine. Intense B/Victoria activity was followed by circulation of genetically diverse A(H1N1)pdm09 viruses that were also antigenically drifted. We measured vaccine effectiveness (VE) in the United States against illness from these emerging viruses.
Methods: We enrolled outpatients aged ≥6 months with acute respiratory illness at 5 sites. Respiratory specimens were tested for influenza by reverse-transcriptase polymerase chain reaction (RT-PCR). Using the test-negative design, we determined influenza VE by virus subtype/lineage and genetic subclades by comparing odds of vaccination in influenza cases versus test-negative controls.
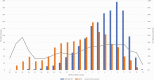
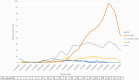
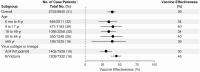
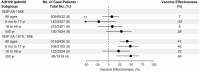
Results: Among 8845 enrollees, 2722 (31%) tested positive for influenza, including 1209 (44%) for B/Victoria and 1405 (51%) for A(H1N1)pdm09. Effectiveness against any influenza illness was 39% (95% confidence interval [CI]: 32-44), 45% (95% CI: 37-52) against B/Victoria and 30% (95% CI: 21-39) against A(H1N1)pdm09-associated illness. Vaccination offered no protection against A(H1N1)pdm09 viruses with antigenically drifted clade 6B.1A 183P-5A+156K HA genes (VE 7%; 95% CI: -14 to 23%) which predominated after January.
Conclusions: Vaccination provided protection against influenza illness, mainly due to infections from B/Victoria viruses. Vaccine protection against illness from A(H1N1)pdm09 was lower than historically observed effectiveness of 40%-60%, due to late-season vaccine mismatch following emergence of antigenically drifted viruses. The effect of drift on vaccine protection is not easy to predict and, even in drifted years, significant protection can be observed.
Keywords: antigenic drift; influenza; test-negative; vaccination; vaccine effectiveness.
Published by Oxford University Press for the Infectious Diseases Society of America 2020.
Figures
References
-
- Centers for Disease Control and Prevention. Weekly U.S. influenza surveillance report. Available at: https://www.cdc.gov/flu/weekly/index.htm. Accessed 21 July 20.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

