Approach to the Virilizing Girl at Puberty
- PMID: 33367768
- PMCID: PMC8063244
- DOI: 10.1210/clinem/dgaa948
Approach to the Virilizing Girl at Puberty
Abstract
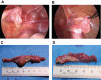
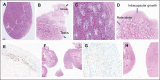
Virilization is the medical term for describing a female who develops characteristics associated with male hormones (androgens) at any age, or when a newborn girl shows signs of prenatal male hormone exposure at birth. In girls, androgen levels are low during pregnancy and childhood. A first physiologic rise of adrenal androgens is observed at the age of 6 to 8 years and reflects functional activation of the zona reticularis of the adrenal cortex at adrenarche, manifesting clinically with first pubic and axillary hairs. Early adrenarche is known as "premature adrenarche." It is mostly idiopathic and of uncertain pathologic relevance but requires the exclusion of other causes of androgen excess (eg, nonclassic congenital adrenal hyperplasia) that might exacerbate clinically into virilization. The second modest physiologic increase of circulating androgens occurs then during pubertal development, which reflects the activation of ovarian steroidogenesis contributing to the peripheral androgen pool. However, at puberty initiation (and beyond), ovarian steroidogenesis is normally devoted to estrogen production for the development of secondary female bodily characteristics (eg, breast development). Serum total testosterone in a young adult woman is therefore about 10- to 20-fold lower than in a young man, whereas midcycle estradiol is about 10- to 20-fold higher. But if androgen production starts too early, progresses rapidly, and in marked excess (usually more than 3 to 5 times above normal), females will manifest with signs of virilization such as masculine habitus, deepening of the voice, severe acne, excessive facial and (male typical) body hair, clitoromegaly, and increased muscle development. Several medical conditions may cause virilization in girls and women, including androgen-producing tumors of the ovaries or adrenal cortex, (non)classical congenital adrenal hyperplasia and, more rarely, other disorders (also referred to as differences) of sex development (DSD). The purpose of this article is to describe the clinical approach to the girl with virilization at puberty, focusing on diagnostic challenges. The review is written from the perspective of the case of an 11.5-year-old girl who was referred to our clinic for progressive, rapid onset clitoromegaly, and was then diagnosed with a complex genetic form of DSD that led to abnormal testosterone production from a dysgenetic gonad at onset of puberty. Her genetic workup revealed a unique translocation of an abnormal duplicated Y-chromosome to a deleted chromosome 9, including the Doublesex and Mab-3 Related Transcription factor 1 (DMRT1) gene.
Learning objectives: Identify the precise pathophysiologic mechanisms leading to virilization in girls at puberty considering that virilization at puberty may be the first manifestation of an endocrine active tumor or a disorder/difference of sex development (DSD) that remained undiagnosed before and may be life-threatening. Of the DSDs, nonclassical congenital adrenal hyperplasia occurs most often.Provide a step-by-step diagnostic workup plan including repeated and expanded biochemical and genetic tests to solve complex cases.Manage clinical care of a girl virilizing at puberty using an interdisciplinary team approach.Care for complex cases of DSD manifesting at puberty, such as the presented girl with a Turner syndrome-like phenotype and virilization resulting from a complex genetic variation.
Keywords: androgen excess; disorders/differences of sex development (DSD); endocrine active tumors; genetic disorders of androgen excess; virilization.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


Similar articles
-
Normal and Premature Adrenarche.Endocr Rev. 2021 Nov 16;42(6):783-814. doi: 10.1210/endrev/bnab009. Endocr Rev. 2021. PMID: 33788946 Free PMC article. Review.
-
Hirsutism and virilism in women.Spec Top Endocrinol Metab. 1984;6:55-93. Spec Top Endocrinol Metab. 1984. PMID: 6084314 Review.
-
Girls with virilisation in childhood: a diagnostic protocol for investigation.J Clin Pathol. 1997 May;50(5):379-83. doi: 10.1136/jcp.50.5.379. J Clin Pathol. 1997. PMID: 9215119 Free PMC article.
-
Case report: Development of central precocious puberty in a girl with late-diagnosed simple virilizing congenital adrenal hyperplasia complicated with Williams syndrome.Front Endocrinol (Lausanne). 2024 Apr 18;15:1352552. doi: 10.3389/fendo.2024.1352552. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38699383 Free PMC article.
-
'Exaggerated adrenarche' in children presenting with premature adrenarche.Clin Endocrinol (Oxf). 1995 Mar;42(3):265-72. doi: 10.1111/j.1365-2265.1995.tb01874.x. Clin Endocrinol (Oxf). 1995. PMID: 7758231
Cited by
-
Adult Height in Girls With Idiopathic Premature Adrenarche: A Cohort Study and Design of a Predictive Model.Front Endocrinol (Lausanne). 2022 Mar 3;13:852422. doi: 10.3389/fendo.2022.852422. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35311244 Free PMC article.
-
Clitoromegaly due to an epidermal inclusion cyst: A case report.Case Rep Womens Health. 2022 Jul 18;35:e00432. doi: 10.1016/j.crwh.2022.e00432. eCollection 2022 Jul. Case Rep Womens Health. 2022. PMID: 35898429 Free PMC article.
-
Understanding Sex Differences in Autoimmune Diseases: Immunologic Mechanisms.Int J Mol Sci. 2025 Jul 23;26(15):7101. doi: 10.3390/ijms26157101. Int J Mol Sci. 2025. PMID: 40806232 Free PMC article. Review.
-
PUBERTAL VIRILIZATION IN AN ADOLESCENT WITH 46, XY DISORDER OF SEXUAL DEVELOPMENT: A NOVEL MUTATION IN NR5A1 GENE.Acta Endocrinol (Buchar). 2023 Jul-Sep;19(3):364-369. doi: 10.4183/aeb.2023.364. Epub 2024 Feb 1. Acta Endocrinol (Buchar). 2023. PMID: 38356974 Free PMC article.
-
Sex hormone influence on female-biased autoimmune diseases hints at puberty as an important factor in pathogenesis.Front Pediatr. 2023 Jan 30;11:1051624. doi: 10.3389/fped.2023.1051624. eCollection 2023. Front Pediatr. 2023. PMID: 36793337 Free PMC article. Review.
References
-
- Miller WL, Fluck CE, Breault DT, Feldman BJ. The adrenal cortex and its disorders. In: Sperling M, ed. Sperling Pediatric Endocrinology. Amsterdam: Elsevier; 2020:425-490.
-
- Lala SV, Strubel N. Ovarian neoplasms of childhood. Pediatr Radiol. 2019;49(11):1463-1475. - PubMed
-
- Marti N, Malikova J, Galván JA, et al. . Androgen production in pediatric adrenocortical tumors may occur via both the classic and/or the alternative backdoor pathway. Mol Cell Endocrinol. 2017;452:64-73. - PubMed

