Mitochondria-targeted therapeutics, MitoQ and BGP-15, reverse aging-associated meiotic spindle defects in mouse and human oocytes
- PMID: 33367783
- PMCID: PMC7891816
- DOI: 10.1093/humrep/deaa300
Mitochondria-targeted therapeutics, MitoQ and BGP-15, reverse aging-associated meiotic spindle defects in mouse and human oocytes
Abstract
Study question: Do mitochondria-targeted therapies reverse ageing- and oxidative stress-induced spindle defects in oocytes from mice and humans?
Summary answer: Exposure to MitoQ or BGP-15 during IVM protected against spindle and chromosomal defects in mouse oocytes exposed to oxidative stress or derived from reproductively aged mice whilst MitoQ promoted nuclear maturation and protected against chromosomal misalignments in human oocytes.
What is known already: Spindle and chromosomal abnormalities in oocytes are more prevalent with maternal aging, increasing the risk of aneuploidy, miscarriage and genetic disorders such as Down's syndrome. The origin of compromised oocyte function may be founded in mitochondrial dysfunction and increased reactive oxygen species (ROS).
Study design, size, duration: Oocytes from young and old mice were treated with MitoQ and/or BGP-15 during IVM. To directly induce mitochondrial dysfunction, oocytes were treated with H2O2, and then treated the MitoQ and/or BGP-15. Immature human oocytes were cultured with or without MitoQ. Each experiment was repeated at least three times, and data were analyzed by unpaired-sample t-test or chi-square test.
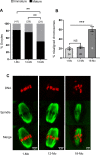
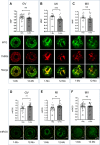
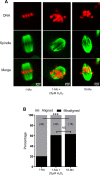
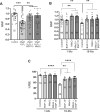
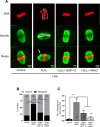
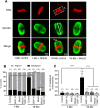
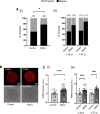
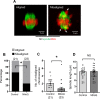
Participants/materials, setting, methods: Immature germinal vesicle (GV) stage oocytes from 1-, 12- and 18-month-old mice were obtained from preovulatory ovarian follicles. Oocytes were treated with MitoQ and/or BGP-15 during IVM. GV-stage human oocytes were cultured with or without MitoQ. Mitochondrial membrane potential and mitochondrial ROS were measured by live-cell imaging. Meiotic spindle and chromosome alignments were visualized by immunofluorescent labeling of fixed oocytes and the 3-dimensional images were analyzed by Imaris.
Main results and the role of chance: MitoQ or BGP-15 during IVM protects against spindle and chromosomal defects in oocytes exposed to oxidative stress and in oocytes from aged mice (P < 0.001). In human oocytes, the presence of MitoQ during IVM promoted nuclear maturation and had a similar positive effect in protecting against chromosomal misalignments (P < 0.001).
Limitations, reasons for caution: Our study identifies two excellent candidates that may help to improve fertility in older women. However, these potential therapies must be tested for efficacy in clinical IVM systems, and undergo thorough examination of resultant offspring in preclinical models before utilization.
Wider implications of the findings: Our results using in-vitro systems for oocyte maturation in both mouse and human provide proof of principle that mitochondrially targeted molecules such as MitoQ and BGP-15 may represent a novel therapeutic approach against maternal aging-related spindle and chromosomal abnormalities.
Study funding/competing interest(s): The project was financially supported by the National Health and Medical Research Council and Australian Research Council, Australia. U.A.-Z. was supported by the Iraqi Higher Education and Scientific Research Ministry PhD scholarship and O.C. was supported by TUBITAK-1059B191601275. M.P.M. consults for MitoQ Inc. and holds patents in mitochondria-targeted therapies. R.L.R. is an inventor on patents relating to the use of BGP-15 to improve gamete quality.
Trial registration number: N/A.
Keywords: aging; chromosome; mitochondria-targeted therapeutics; oocyte; spindle.
© The Author(s) 2020. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures
References
-
- Angell RR. Aneuploidy in older women. Higher rates of aneuploidy in oocytes from older women. Hum Reprod 1994;9:1199–1200. - PubMed
-
- Baird DT, Collins J, Egozcue J, Evers LH, Gianaroli L, Leridon H, Sunde A, Templeton A, Van Steirteghem A, Cohen J. et al. ; ESHRE Capri Workshop Group. Fertility and ageing. Hum Reprod Update 2005;11:261–276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

